This is a preprint.
Pharmacokinetics of dexamethasone in tuberculosis meningitis
- PMID: 40791718
- PMCID: PMC12338885
- DOI: 10.1101/2025.07.14.25331510
Pharmacokinetics of dexamethasone in tuberculosis meningitis
Abstract
Introduction: Dexamethasone is recommended as adjunctive therapy for tuberculosis meningitis (TBM). Co-administration with rifampicin is expected to reduce dexamethasone exposure in TBM, an effect that may be more pronounced with the higher rifampicin doses currently being evaluated in clinical trials.
Methods: This pharmacokinetic study was nested in a randomised controlled trial comparing the safety of high-dose rifampicin (oral, 35 mg/kg; intravenous, 20 mg/kg) plus linezolid, with or without aspirin, vs standard-dose rifampicin (10 mg/kg) for adults with HIV-associated TBM. All participants received adjunctive oral dexamethasone every 12 hours starting at a dose of 0.4 mg/kg/day. Dexamethasone concentrations were measured on intensively sampled plasma on day 3 after study enrolment and analysed using nonlinear mixed-effects modelling.
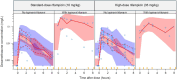
Results: In total, 261 dexamethasone concentrations from 43 participants were available for model development. Eight (18%) participants were on efavirenz-based ART and five (11%) were on a lopinavir/ritonavir-based regimen. The median duration of rifampicin therapy at the time of pharmacokinetic sampling was 4 days (range: 0-7). Dexamethasone pharmacokinetics was best described by a one-compartment disposition model with first-order absorption and elimination. Typical oral clearance (CL/F) was 131 L/h, reduced to 11.5 L/h with concomitant lopinavir/ritonavir. High-dose rifampicin had no significant additional effect on dexamethasone pharmacokinetic parameters compared with the standard-dose.
Conclusions: In adults with HIV-associated TBM, there was high dexamethasone clearance, likely related to a drug-drug interaction with rifampicin. High-dose rifampicin had no additional effect on dexamethasone exposure.
Keywords: Dexamethasone; Drug-Drug Interaction; HIV; Lopinavir/Ritonavir; NONMEM; Pharmacokinetics; Rifampicin; Tuberculous Meningitis.
Conflict of interest statement
CONFLICT OF INTEREST All authors declare no conflict of interest.
Figures


References
-
- Dodd PJ, Osman M, Cresswell F V., et al. The global burden of tuberculous meningitis in adults: A modelling study. PLOS Global Public Health 2021; 1:e0000069. Available at: https://dx.plos.org/10.1371/journal.pgph.0000069. - DOI - PMC - PubMed
-
- Thwaites GE, Bang ND, Dung NH, et al. Dexamethasone for the Treatment of Tuberculous Meningitis in Adolescents and Adults. New England Journal of Medicine 2004; 351:1741–1751. Available at: http://www.nejm.org/doi/abs/10.1056/NEJMoa040573. - DOI - PubMed
-
- Donovan J, Bang ND, Imran D, et al. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. New England Journal of Medicine 2023; 389:1357–1367. Available at: http://www.nejm.org/doi/10.1056/NEJMoa2216218. - DOI - PMC - PubMed
-
- Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database of Systematic Reviews 2016; 2016. Available at: http://doi.wiley.com/10.1002/14651858.CD002244.pub4. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
