Restoration of tendon repair microenvironment by grapefruit exosome-loaded microneedle system for tendinopathy therapy
- PMID: 40791857
- PMCID: PMC12336195
- DOI: 10.3389/fbioe.2025.1615650
Restoration of tendon repair microenvironment by grapefruit exosome-loaded microneedle system for tendinopathy therapy
Abstract
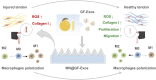
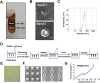
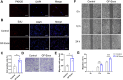
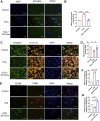
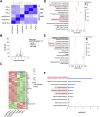
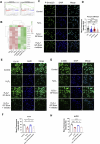
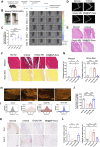
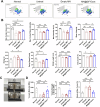
Tendinitis repair remains challenging due to the limited self-renewal capacity of tenocytes and persistent inflammatory microenvironment. Conventional therapies remain limited by systemic drug toxicity and fail to coordinate immunomodulation with matrix remodeling. Plant-derived extracellular vesicles have demonstrated tissue repair potential owing to their unique bioactive components and exceptional cross-species compatibility. Nevertheless, their therapeutic role in tendon matrix regeneration remains underexplored. Here, we developed a grapefruit-derived exosome-loaded microneedle patch (MN@GF-Exos) to synergistically restored tendon structure and functions. Grapefruit-derived exosomes (GF-Exos) were loaded into dissolvable hyaluronic acid microneedles (MNs) for sustained release. GF-Exos reversed oxidative stress in tenocytes, enhancing cellular proliferation and migration, restoring collagen I synthesis, and polarizing macrophages toward M2-repair phenotypes. Transcriptomics revealed GF-Exos modulated cytokine-cytokine receptor interactions, suppressing inflammation-related pathways and activating ECM organization genes. In collagenase-induced tendinopathy mice, MN@GF-Exos enhanced gait recovery and extracellular matrix remodeling. Histology confirmed reduced fibrosis without ectopic ossification. Systemic safety was validated by unchanged organ histology and within-normal-limits serum biomarkers. This dual-functional system leverages plant exosomes' multi-component synergy and MN's spatiotemporal control, offering a translatable strategy for chronic tendon regeneration.
Keywords: grapefruit-derived exosome; macrophage polarization; microneedles; oxidative stress; tendinopathy.
Copyright © 2025 Zhang, Zhang, Zhang, Mu, Juma, Li, Li, Guo and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats.J Orthop Translat. 2025 Jun 5;53:63-81. doi: 10.1016/j.jot.2025.04.015. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40529900 Free PMC article.
-
hucMSC-derived exosomes targeting macrophage polarization attenuate systemic inflammation in T1DM via INS/SOD1 delivery.Stem Cell Res Ther. 2025 Jul 18;16(1):384. doi: 10.1186/s13287-025-04521-0. Stem Cell Res Ther. 2025. PMID: 40682139 Free PMC article.
-
Personalized human umbilical cord mesenchymal stem cell-derived exosome pre-treatment based on the simulation of scar microenvironment characteristics: a promising approach for early scar treatment.Mol Biol Rep. 2025 Jul 23;52(1):747. doi: 10.1007/s11033-025-10794-8. Mol Biol Rep. 2025. PMID: 40699397
-
Construction and applications of exosome-microneedle integrated systems.Int J Pharm X. 2025 Jul 15;10:100360. doi: 10.1016/j.ijpx.2025.100360. eCollection 2025 Dec. Int J Pharm X. 2025. PMID: 40727683 Free PMC article. Review.
-
Molecular and therapeutic effects of mesenchymal stem cell-derived exosomes on autoimmune diseases.World J Stem Cells. 2025 Jul 26;17(7):107202. doi: 10.4252/wjsc.v17.i7.107202. World J Stem Cells. 2025. PMID: 40740529 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

