Inhibition of the TLR4/RAGE pathway by clearance of extracellular HMGB1 is a potential therapeutic target for radiation-damaged salivary glands
- PMID: 40791981
- PMCID: PMC12335976
- DOI: 10.1016/j.reth.2025.07.004
Inhibition of the TLR4/RAGE pathway by clearance of extracellular HMGB1 is a potential therapeutic target for radiation-damaged salivary glands
Abstract
Introduction: We recently developed a new therapy using effective-mononuclear cells (E-MNCs) and demonstrated its efficacy in treating radiation-damaged salivary glands (SGs). The activity of E-MNCs in part involves constituent immunoregulatory -CD11b/macrophage scavenger receptor 1(Msr1)-positive-M2 macrophages, which exert anti-inflammatory and tissue-regenerating effects via phagocytic clearance of extracellular high mobility group box 1 (HMGB1). Focusing on the phenomena, this study investigated significance of regulating the HMGB1/toll-like receptor 4 (TLR4)/receptor for advanced glycation end products (RAGE) signaling pathway in the treatment of SG dysfunction caused by radiation damage.
Methods: E-MNCs were transplanted into radiation-damaged mice SGs, and changes of TLR4/RAGE expression were observed. Furthermore, the activation of downstream signals was investigated in both intact SGs and cultured SG epithelial cells after irradiation. Subsequently, TLR4-knock-out (KO) mice were employed to examine how HMGB1/TLR4/RAGE signaling affected damage progression.
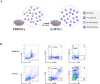
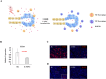
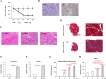
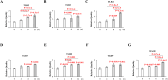
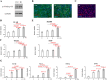
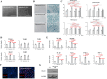
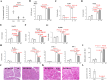
Results: Expression of both TLR4 and RAGE was diminished in ductal cells and macrophages/vascular endothelial cells of damaged SGs with E-MNC transplantation, respectively. Meanwhile, expression of TLR2/4 and RAGE in damaged SGs markedly increased in association with extracellular HMGB1 accumulation. Downstream signals were activated, and intranuclear localization of phospho-nuclear factor-kappa B (p-NF-KB) in ductal cells and production of IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were observed. Additionally, culture supernatant of irradiated cultured SG epithelial cells contained damaged associated molecular pattern (DAMP)/senescence-associated secretory phenotype (SASP) factors. Treatment of cultured SG epithelial cells with this supernatant activated TLR4 signaling pathway and induced cellular senescence. In TLR4-KO mice, onset of radiogenic SG dysfunction was markedly delayed. However, TLR2/RAGE signalings were alternatively activated, and SG function was impaired.
Conclusions: Clearance of DAMPs such as HMGB1 may attenuate sterile inflammation in damaged SGs via suppression of the TLR4/RAGE signaling pathway. This cellular mechanism may have significant implications for the development of future cell-based regenerative therapies.
© 2025 The Author(s).
Conflict of interest statement
Author MS is the CEO of CellAxia Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Alajbeg I., Falcão D.P., Tran S.D., Martín-Granizo R., Lafaurie G.I., Matranga D., et al. Intraoral electrostimulator for xerostomia relief: a long-term, multicenter, open-label, uncontrolled, clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(6):773–781. - PubMed
-
- Baum B.J., Kok M., Tran S.D., Yamano S. The impact of gene therapy on dentistry: a revisiting after six years. J Am Dent Assoc. 2002;133(1):35–44. - PubMed
-
- Baum B.J., Tran S.D. Synergy between genetic and tissue engineering: creating an artificial salivary gland. Periodontol. 2000. 2006;41:218–223. - PubMed
-
- Kagami H. The potential use of cell-based therapies in the treatment of oral diseases. Oral Dis. 2015;21(5):545–549. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

