GCK inhibition enhances iberdomide antimyeloma effects by promoting IKZF1 degradation via a CRBN-independent mechanism
- PMID: 40792016
- PMCID: PMC12335957
- DOI: 10.1016/j.bneo.2025.100130
GCK inhibition enhances iberdomide antimyeloma effects by promoting IKZF1 degradation via a CRBN-independent mechanism
Abstract
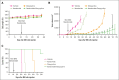
Our recent study identifies germinal center kinase (GCK) as a novel therapeutic target in RAS-mutated multiple myeloma (MM). Inhibiting GCK downregulates critical transcriptional factors, notably IKZF1/3, BCL-6, and c-MYC proteins, leading to MM cell growth inhibition and cell death. Distinct from immunomodulatory drug (IMiD)-induced IKZF1/3 degradation, GCK inhibition triggers IKZF1/3 proteolysis through a cereblon (CRBN) E3 ligase-independent mechanism. Here, we demonstrated that GCK inhibition overcomes IMiD resistance in MM. An isogenic subline of MM.1S cells with acquired lenalidomide resistance remains sensitive to GCK inhibition-induced IKZF1/3 downregulation and cell growth inhibition. Consistently, the CRBN-resistant IKZF1 Q146H mutant maintains sensitivity to GCK inhibitor-induced degradation, similar to the IKZF1 wild-type protein, suggesting a CRBN-independent protein degradation. In accordance with the distinct IKZF1/3 degradation mechanisms, GCK silencing enhances iberdomide-induced IKZF1/3 and c-MYC downregulation and MM growth inhibition. More importantly, the combination of a GCK inhibitor with iberdomide exhibited synergistic anti-MM effects in a panel of MM cell lines and primary plasma cells. The synergistic effects were confirmed in an MM xenograft mouse model, in which combining GCK silencing and iberdomide resulted in significantly enhanced tumor inhibition and prolonged mice survival compared to single treatments. These findings underscore GCK as a promising therapeutic target for bypassing IMiD resistance in MM. Combining GCK inhibition with iberdomide could provide a novel strategy to manage relapsed or refractory patients with multidrug resistance, especially after the exhaustion of immunotherapy.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S. Li is currently a full-time employee of Bristol Myers Squibb. C.M. is a full-time employee of Sanofi. S. Lentzsch reports Caelum Biosciences patents and royalties on CAEL-101; advisory board fees from Pfizer, GlaxoSmithKline, Karyopharm, Regeneron, Janssen, and Sanofi; honoraria from Regeneron, PeerView, Clinical Care Options, Medscape, and Springer Healthcare; membership on an entity's board of directors or advisory committees for Regeneron, Janssen, and Bristol Myers Squibb; and research funding from Sanofi and Zentalis. The remaining authors declare no competing financial interests.
Figures


Similar articles
-
Targeting degradation of IKZF1 and IKZF3 through modulation of the E3 ligase substrates in the context of cellular therapies for multiple myeloma.Biomark Res. 2025 Aug 15;13(1):105. doi: 10.1186/s40364-025-00825-8. Biomark Res. 2025. PMID: 40817326 Free PMC article. Review.
-
A novel cereblon variant with both exon 8 and 10 deletions in newly diagnosed and relapsed multiple myeloma.Blood Neoplasia. 2025 Apr 4;2(3):100099. doi: 10.1016/j.bneo.2025.100099. eCollection 2025 Aug. Blood Neoplasia. 2025. PMID: 40612717 Free PMC article.
-
Transcriptional Heterogeneity Overcomes Super-Enhancer Disrupting Drug Combinations in Multiple Myeloma.Blood Cancer Discov. 2024 Jan 8;5(1):34-55. doi: 10.1158/2643-3230.BCD-23-0062. Blood Cancer Discov. 2024. PMID: 37767768 Free PMC article.
-
Serum Cereblon (CRBN) Levels Predict Long Term Post- Lenalidomide-Dexamethasone Survival in Multiple Myeloma (MM) Patients and Correlate with Disease Characteristics.Int J Mol Sci. 2025 Jun 30;26(13):6341. doi: 10.3390/ijms26136341. Int J Mol Sci. 2025. PMID: 40650115 Free PMC article.
-
Management of Adverse Events Associated with Pomalidomide-Based Combinations in Patients with Relapsed/Refractory Multiple Myeloma.Cancers (Basel). 2024 Feb 29;16(5):1023. doi: 10.3390/cancers16051023. Cancers (Basel). 2024. PMID: 38473381 Free PMC article. Review.
References
-
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

