PIEZO1 mechanical insensitivity in generalized lymphatic dysplasia with the potential for pharmacological rescue
- PMID: 40792030
- PMCID: PMC12337661
- DOI: 10.1016/j.isci.2025.113110
PIEZO1 mechanical insensitivity in generalized lymphatic dysplasia with the potential for pharmacological rescue
Abstract
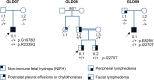
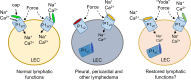
PIEZO1 variants have been associated with generalized lymphatic dysplasia (GLD) through mechanisms involving reduced PIEZO1 expression. Here, we report variants where the mechanism involves reduced channel mechanical sensitivity. Two of the variants encode amino acid changes in the channel's cap structure (Ile2270Thr and Arg2335Gln), one in the ninth transmembrane helical unit (THU) below the cap (Gly1978Asp) and one in the fifth THU distant from the cap (Glu829Val). Patch-clamp studies of the cap and sub-cap variant channels revealed abolished or reduced channel mechanical sensitivity with the possibility to activate the channels and partly rescue mechanical sensitivity by the small molecule Yoda1. The potency of Yoda1 at the variant channels was less than at the wild-type channel, but chemical synthesis of Yoda1 analogs revealed a molecule with improved potency. The data suggest cases of GLD in which there is decreased channel mechanical sensitivity and the potential to reduce dysfunction pharmacologically.
Keywords: Cell biology; Pharmacology.
© 2025 The Author(s).
Conflict of interest statement
D.J.B. and R.F. are partners of CalTIC GmbH, a pharmaceutical startup company with a mission to develop ion channel modulators as classes of medicines.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

