Circulating miRNAs as liquid biopsy biomarkers for diagnosis in patients with colorectal cancer: a systematic review and meta-analysis
- PMID: 40792072
- PMCID: PMC12336027
- DOI: 10.3389/fgene.2025.1574586
Circulating miRNAs as liquid biopsy biomarkers for diagnosis in patients with colorectal cancer: a systematic review and meta-analysis
Abstract
Objective: Colorectal cancer (CRC) remains a leading cause of cancer-related mortality worldwide, emphasizing the need for noninvasive and reliable diagnostic tools. Circulating microRNAs (miRNAs) have emerged as promising liquid biopsy biomarkers for CRC detection. This meta-analysis aimed to evaluate the diagnostic accuracy of blood- and saliva-derived miRNAs in CRC, assessing their sensitivity, specificity, and overall clinical potential.
Methods: A comprehensive literature search was conducted across PubMed, Web of Science, and Scopus to identify relevant studies published between 2004 and 2024. Eligible studies included those that evaluated miRNA expression in plasma, serum, or saliva of CRC patients. A random-effects model was applied to calculate pooled sensitivity, specificity, diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC). Heterogeneity was assessed using Cochrane's Q test and I2 statistics, and risk of bias was evaluated using the QUADAS-2 tool.
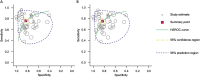
Results: A total of 37 studies with 2,775 patients were included in this meta-analysis. The pooled diagnostic performance demonstrated an AUC of 0.87 for combined blood- and saliva-derived miRNAs and 0.86 for blood-derived miRNAs alone, with both categories showing a sensitivity of 0.76 and specificity of 0.83. The diagnostic likelihood ratio (DLR) analysis yielded DLR positive values > 4 and DLR negative values < 0.3, indicating strong discriminatory ability. The DOR was 15.98 for combined blood- and saliva-derived miRNAs and 15.49 for blood-derived miRNAs alone, highlighting their comparable diagnostic potential. These findings suggest that circulating miRNAs serve as reliable biomarkers for CRC detection.
Conclusion: This meta-analysis supports the diagnostic utility of circulating miRNAs as noninvasive biomarkers for CRC detection, with saliva-derived miRNAs offering a potential complementary role. Blood-based miRNA analysis demonstrated high diagnostic accuracy, and the integration of saliva-derived miRNAs slightly improved AUC. Future research should focus on standardizing miRNA panels and validation in larger, independent cohorts to facilitate their clinical application in CRC screening and early detection.
Keywords: biomarker; circulating biomarker; colorectal cancer; liquid biopsy; saliva diagnostics.
Copyright © 2025 Schwab and Nonaka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Arroyo J. D., Chevillet J. R., Kroh E. M., Ruf I. K., Pritchard C. C., Gibson D. F., et al. (2011). Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. U. S. A. 108 (12), 5003–5008. 10.1073/pnas.1019055108 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

