Respiratory microbiota diversity and composition in recurrent protracted bacterial bronchitis: a cross-sectional study
- PMID: 40792104
- PMCID: PMC12336228
- DOI: 10.3389/fcimb.2025.1524116
Respiratory microbiota diversity and composition in recurrent protracted bacterial bronchitis: a cross-sectional study
Abstract
Introduction: Recurrent protracted bacterial bronchitis (RPBB) is a significant risk factor for bronchiectasis in children, characterized by multiple episodes of protracted bacterial bronchitis (PBB) annually. With an increasing global incidence, a detailed understanding of RPBB's pathophysiology is essential, particularly regarding the role of lung microbiota.
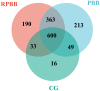
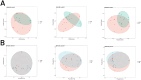
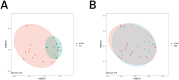
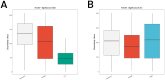
Methods: This cross-sectional study recruited 39 children from Jinhua Maternal and Child Health Hospital between January 2021 and December 2022, including 18 with PBB, 11 with RPBB, and 10 as controls. Bronchoscopy with bronchoalveolar lavage (BAL) was performed to collect lung microbiota samples, which were analyzed using 16S rDNA sequencing. Microbial diversity and composition differences among groups were assessed using alpha and beta diversity metrics, PERMANOVA, and Linear Discriminant Analysis Effect Size (LEfSe), with statistical significance set at P < 0.05.
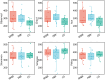
Results: RPBB patients exhibited a distinct lung microbiota composition compared to controls, characterized by an increased abundance of pathogens such as Acinetobacter and Mycoplasma, alongside a reduction in beneficial genera like Streptococcus and Granulicatella. The RPBB group also demonstrated greater overall microbiota diversity, indicating dysbiosis that may contribute to disease severity and persistent respiratory symptoms.
Conclusion: This study revealed significant alterations in the lung microbiota of children with RPBB, suggesting that microbial imbalance could play a crucial role in disease pathogenesis. These findings highlight the importance of targeted prevention and therapeutic strategies aimed at restoring microbiota balance to improve pediatric respiratory health.
Keywords: children; dysbiosis; lung microbiota; microbial imbalance; recurrent protracted bacterial bronchitis.
Copyright © 2025 Xu, Ji, Lin, Chen and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Mucolytics for children with chronic suppurative lung disease.Cochrane Database Syst Rev. 2025 Mar 28;3(3):CD015313. doi: 10.1002/14651858.CD015313.pub2. Cochrane Database Syst Rev. 2025. PMID: 40152354
-
Oral bacterial community dynamics during induction of gingival inflammation.Front Cell Infect Microbiol. 2025 Jun 16;15:1597690. doi: 10.3389/fcimb.2025.1597690. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40589868 Free PMC article.
-
Microbiome and metabolome patterns after lung transplantation reflect underlying disease and chronic lung allograft dysfunction.Microbiome. 2024 Oct 9;12(1):196. doi: 10.1186/s40168-024-01893-y. Microbiome. 2024. PMID: 39385282 Free PMC article.
-
Bronchoscopy-guided antimicrobial therapy for cystic fibrosis.Cochrane Database Syst Rev. 2024 May 3;5(5):CD009530. doi: 10.1002/14651858.CD009530.pub5. Cochrane Database Syst Rev. 2024. PMID: 38700027 Free PMC article.
-
An observational study of the lung microbiome and lung function in young children with cystic fibrosis across two countries with differing antibiotic practices.Microb Pathog. 2025 Aug;205:107628. doi: 10.1016/j.micpath.2025.107628. Epub 2025 Apr 25. Microb Pathog. 2025. PMID: 40288428
References
-
- Chen Y. W., Li S. W., Lin C. D., Huang M. Z., Lin H. J., Chin C. Y., et al. (2020). Fine particulate matter exposure alters pulmonary microbiota composition and aggravates pneumococcus-induced lung pathogenesis. Front. Cell Dev. Biol. 8, 570484. doi: 10.3389/fcell.2020.570484, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

