Advancements in biomaterials and bioactive solutions for lumbar spine fusion cages: Current trends and future perspectives
- PMID: 40792114
- PMCID: PMC12337673
- DOI: 10.1016/j.bioactmat.2025.07.035
Advancements in biomaterials and bioactive solutions for lumbar spine fusion cages: Current trends and future perspectives
Abstract
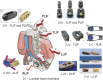
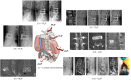
Spinal fusion is considered today as the last treatment option for different spinal conditions, such as degenerative and infectious illnesses. It consists of fusing two or more vertebrae to obtain reinforcement/fixation based on several methods used to sustain osteosynthesis and grafting, such as cage insertion in the intervertebral space, which provides an important level of mechanical stability, impacting only a low amount of the natural biomechanics of the spine and facilitating the implant bony ingrowth. This review paper first explores the background of intervertebral fusion, emphasizing medical applications and material properties of interbody fusion cages. It then provides a brief historical overview and discusses antibacterial efficacy-related issues. Additionally, some of the most met-in-clinical practice lumbar interbody cages with a detailed description of their geometry and examples of clinical trials performed worldwide are provided. The biomaterials used in lumbar cage manufacture are comprehensively described. In the last part of this review paper, special attention is devoted to prospective biomaterials and coatings for spine fusion cages. Firstly, the rationale for using Mg-based alloys or high osteogenic polycaprolactone as biodegradable and bioresorbable alternatives in the spinal cage industry, addressing the clinical limitations of traditional Ti alloys and polyether ether ketone, is provided. Then, a more conservative approach, focusing on the use of bioactive or antibacterial coatings on the already certified biomaterials, is presented as a second alternative to the existing products on the market. Relevant literature studies are reviewed, and the osteointegrative, bioactive, or antibacterial character of the coatings is explained. Finally, our review identifies current clinical limitations and offers future perspectives that will provide better bioactive solutions, improving the existing biomaterials.
Keywords: Bioactive solutions; Biodegradable cages; Clinical trials; High osteogenic polycaprolactone; Mg-based alloys; Spinal fusion.
© 2025 The Authors.
Conflict of interest statement
Julietta V. Rau is an associate editor board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. Iulian Antoniac is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures

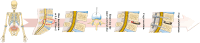




Similar articles
-
Maximizing screw length in expandable lateral lumbar interbody spacers with integrated fixation may obviate the need for supplemental pedicle screws.Spine J. 2025 Jul;25(7):1564-1573. doi: 10.1016/j.spinee.2025.01.035. Epub 2025 Jan 30. Spine J. 2025. PMID: 39890027
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
Complications related to osteobiologics use in spine surgery: a systematic review.Spine (Phila Pa 1976). 2010 Apr 20;35(9 Suppl):S86-104. doi: 10.1097/BRS.0b013e3181d81ef2. Spine (Phila Pa 1976). 2010. PMID: 20407355
-
Influence of the geometric and material properties of lumbar endplate on lumbar interbody fusion failure: a systematic review.J Orthop Surg Res. 2022 Apr 10;17(1):224. doi: 10.1186/s13018-022-03091-8. J Orthop Surg Res. 2022. PMID: 35399075 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Lim K.-M., Park T.-H., Lee S.-J., Park S.-J. Design and biomechanical verification of additive manufactured composite spinal cage composed of porous titanium cover and PEEK body. Appl. Sci. 2019;9:4258. doi: 10.3390/app9204258. - DOI
-
- Warren J.M., Hey L.A., Mazzoleni A.P. A finite element study of the relationship between upper body weight and the loads experienced by the human Lumbosacral spine, and fusion instrumentation, in a standing upright posture. Biomed. Eng. Adv. 2021;2 doi: 10.1016/j.bea.2021.100023. - DOI
-
- Brinjikji W., Luetmer P.H., Comstock B., Bresnahan B.W., Chen L.E., Deyo R.A., Halabi S., Turner J.A., Avins A.L., James K., et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J. Neuroradiol. 2015;36:811–816. doi: 10.3174/ajnr.A4173. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

