The SAP130/Mincle axis was involved in sevoflurane-induced neuronal death and microglial activation in juvenile mice
- PMID: 40792209
- PMCID: PMC12336192
- DOI: 10.3389/fphar.2025.1647329
The SAP130/Mincle axis was involved in sevoflurane-induced neuronal death and microglial activation in juvenile mice
Abstract
Introduction: Sevoflurane is widely used in pediatric anesthesia and has raised concerns for years regarding its neurotoxic effects on the developing brain. Studies have shown that sevoflurane can lead to neuronal cell death and neuroinflammation, which further contribute to sevoflurane-induced neurotoxicity manifested as delirium or cognitive deficits. However, the molecular mechanism remains poorly understood. A factor of interest is Sin3A-associated protein 130 (SAP130), which can be released by dead or damaged cells and trigger sterile inflammation, exacerbating tissue damage by activating the macrophage-inducible C-type lectin (Mincle) receptor. However, whether the SAP130/Mincle axis is involved in sevoflurane-induced neurotoxicity remains unknown.
Methods: Using a young murine sevoflurane exposure model and a primary neuron-microglia co-culture system, we examined changes in neuronal cell death, microglial activation, cytokine production, and the expression levels of SAP130- and Mincle-signaling-associated proteins after sevoflurane exposure. We then applied SAP130-neutralizing antibody and the Syk inhibitor piceatannol to assess the impact of inhibiting the Mincle pathway on microglial activation and sevoflurane-induced neurotoxicity.
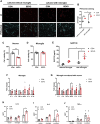
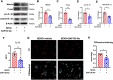
Results: The results demonstrated that sevoflurane exposure increased the number of dead neurons with SAP130 upregulation and induced microglial activation with cytokine production in the hippocampus. These changes occurred only in the neuron-microglia co-culture system but not in neuron or microglia monoculture. Neutralizing SAP130 or pharmacologically inhibiting syk diminished microglial activation and neuronal cell death by suppressing the SAP130/Mincle signaling pathway.
Discussion: These findings suggest that the SAP130/Mincle axis plays a crucial role in neuronal death and microglial activation in sevoflurane-induced neurotoxicity. Targeting this axis emerges as a potential therapeutic strategy to mitigate the neurotoxic effects of sevoflurane.
Keywords: Mincle; SAP130; microglia; neuroinflammation; neuron; sevoflurane.
Copyright © 2025 Zhou, Chen, Zhang, Shu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JL declared a shared affiliation with the authors to the handling editor at the time of review.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

