mTORC1-selective inhibitors rescue cellular phenotypes in TSC iPSC-derived neurons
- PMID: 40792287
- PMCID: PMC12337281
- DOI: 10.3389/fnins.2025.1595880
mTORC1-selective inhibitors rescue cellular phenotypes in TSC iPSC-derived neurons
Abstract
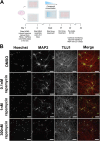
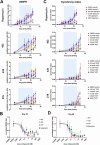
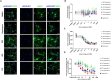
The mechanistic target of rapamycin (mTOR) pathway plays an important role in regulating multiple cellular processes, including cell growth, autophagy, proliferation, protein synthesis, and lipid synthesis, among others. Given the central role of this pathway in multiple cellular processes, it is not surprising that mTOR pathway dysregulation is a key mechanism underlying several neurological disorders, including Tuberous Sclerosis Complex (TSC). TSC patients typically present with pathogenic variants in the TSC1 or TSC2 genes, which encode proteins forming a complex that plays an important role in modulating mTOR activity. We previously reported cellular and functional deficits in induced pluripotent stem cell (iPSC)-derived neurons from TSC patients. These deficits were reversed by inhibiting mTOR activity using rapamycin treatment, revealing the role of mTOR signaling in the regulation of cell morphology and hyperexcitability phenotypes in TSC patient-derived neurons. However, chronic rapamycin treatment inhibits both mTORC1 and mTORC2 activity and its clinical use is associated with significant side effects. With the development of novel mTORC1-selective compounds, we aimed to assess whether selective inhibition of mTORC1 likewise reversed the cellular and functional deficits found in TSC patient-derived neurons. Our results indicate that the novel, selective mTORC1 inhibitors nearly fully reversed the cellular and functional deficits of TSC2 -/ - iPSC-derived neurons in a fashion and magnitude similar to rapamycin, as they all reversed and near-normalized their neuronal hyperexcitability and abnormal morphology as compared to the DMSO-treated cells. These data suggest that mTORC1-specific compounds could provide clinical therapeutic benefit similar to rapamycin without the same side effects.
Keywords: TSC2; hyperexcitability; iPSC-derived neurons; mTOR; mTORC1; mTORC2; soma size.
Copyright © 2025 Buttermore, Srinivasan, Jumo, Swanson, O’Kelly, Makhortova, Sahin and Tzannis.
Conflict of interest statement
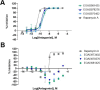
ST was employed by Aeovian Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Aeovian Pharmaceuticals, Inc. The funder had the following involvement in the study: funding from Aeovian supported the development of the novel compounds and the AlphaLISA data presented in Figure 1. Beyond this, Aeovian was involved in the selection of compounds to test on the iPSC-derived neuron assays, the selection of doses tested, and the writing and editing of the manuscript.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

