Giardia duodenalis in Rodents: A Global Systematic Review and Meta-Analysis
- PMID: 40792600
- PMCID: PMC12340709
- DOI: 10.1002/vms3.70546
Giardia duodenalis in Rodents: A Global Systematic Review and Meta-Analysis
Abstract
Background: Giardia duodenalis (also known as G. lamblia or G. intestinalis) is a globally distributed protozoan with zoonotic potential. This systematic review and meta-analysis aimed to determine the global molecular prevalence and genotypic distribution of G. duodenalis in rodents, based exclusively on studies using molecular diagnostic techniques.
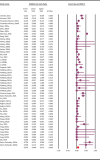
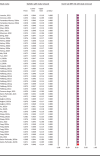
Methods: A comprehensive literature search up to 15 October 2024, identified 23 eligible studies encompassing 54 datasets and 5971 rodent samples from 10 countries across three continents. Prevalence estimates were pooled using a random-effects model, and heterogeneity was assessed via the I2 statistic. Assemblage and sub-assemblage distributions were analysed across rodent species and geographic regions.
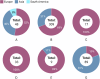
Results: The pooled molecular prevalence of G. duodenalis in rodents was 7.4% (95% CI: 4.8-11.4%), with chinchillas (36.9%) and porcupines (23.1%) showing the highest infection rates. Rodents were found to harbour six assemblages (A-E, G) and four sub-assemblages (AI, AII, BIII, BIV) of G. duodenalis, with marked geographic variation. The highest pooled prevalence was observed in Europe (17.9%; 95% CI: 9.8-30.5), where assemblages C, D, G, and most occurrences of E, B, and A were reported. Assemblages C and D were entirely absent in Asia. In contrast, most reports of the rodent-specific assemblage G originated from Asia. South America (represented solely by Brazil) reported only assemblage A. China contributed the largest dataset (n = 25) and sample size (n = 4009), exhibiting high genetic diversity (A, B, E, G). Belgium also showed notable diversity (A, B, C, E), with assemblage B being the most prevalent in both countries. Assemblage D was found exclusively in Romania, while assemblage C was reported only in Belgium and Italy. Notably, the highest assemblage diversity was observed in chinchillas (five: A-E), squirrels (four: A, B, E, G), and rats (three: A, B, G).
Conclusions: Although various rodent species, especially chinchillas, mice, porcupines, rats, squirrels, and voles, carry G. duodenalis zoonotic assemblages (A and B), the overall molecular prevalence in rodents remains relatively low. Due to significant limitations in sampling design, methodological heterogeneity, limited ecological data, and unknown host health status, current evidence is insufficient to confirm rodents as major zoonotic reservoirs. Standardised, large-scale molecular studies are needed to clarify the epidemiological role of rodents in G. duodenalis transmission.
Keywords: Giardia duodenalis; assemblage; meta‐analysis; molecular prevalence; rodents; systematic review.
© 2025 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest
Figures



Similar articles
-
A Global Systematic Review and Meta-Analysis of Giardia duodenalis in Rabbits: Epidemiology, Genetic Diversity and Possible Zoonotic Concerns.Vet Med Sci. 2025 Jan;11(1):e70176. doi: 10.1002/vms3.70176. Vet Med Sci. 2025. PMID: 39692048 Free PMC article.
-
Diagnostic Accuracy of Various Genetic Markers (tpi, gdh, and bg) for Prevalence and Genotyping of Giardia duodenalis in Human Samples: A Comparative Global Systematic Review and Meta-Analysis.Acta Parasitol. 2025 Jul 14;70(4):161. doi: 10.1007/s11686-025-01101-3. Acta Parasitol. 2025. PMID: 40660056
-
Molecular prevalence of Giardia duodenalis in domestic pig (Sus domesticus) and captive wild boar (Sus scrofa) in China: A systematic review and meta-analysis.Vet Parasitol Reg Stud Reports. 2025 Jan;57:101151. doi: 10.1016/j.vprsr.2024.101151. Epub 2024 Nov 6. Vet Parasitol Reg Stud Reports. 2025. PMID: 39855845
-
Prevalence, genetic diversity, and zoonotic potential of Giardia duodenalis in New and Old World Camelids: A comparative systematic review and meta-analysis.Comp Immunol Microbiol Infect Dis. 2025 Apr;118:102316. doi: 10.1016/j.cimid.2025.102316. Epub 2025 Feb 6. Comp Immunol Microbiol Infect Dis. 2025. PMID: 39947119
-
Global prevalence of Giardia infection in nonhuman mammalian hosts: A systematic review and meta-analysis of five million animals.PLoS Negl Trop Dis. 2025 Apr 24;19(4):e0013021. doi: 10.1371/journal.pntd.0013021. eCollection 2025 Apr. PLoS Negl Trop Dis. 2025. PMID: 40273200 Free PMC article.
References
-
- Asghari, A. , Motazedian M. H., Asgari Q., et al. 2022. “Occurrence, Genetic Characterization, and Zoonotic Importance of Giardia duodenalis in Various Species of Rodents (Mus musculus, Rattus norvegicus, and Rattus rattus).” Comparative Immunology, Microbiology and Infectious Diseases 85: 101812. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

