Cell-Free Production of Soybean Leghemoglobins and Nonsymbiotic Hemoglobin
- PMID: 40792658
- PMCID: PMC12455655
- DOI: 10.1021/acssynbio.5c00197
Cell-Free Production of Soybean Leghemoglobins and Nonsymbiotic Hemoglobin
Abstract
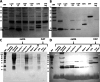
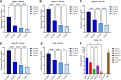
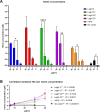
Hemoglobins are heme proteins and are present in certain microorganisms, higher plants, and mammals. Two types of hemoglobin are found in legume nodules: leghemoglobin (LegH) or symbiotic and nonsymbiotic (nsHb). LegHs occur in high amounts in legume roots, and together with bacteroides, are responsible for the nitrogen fixation process. nsHb Class 1 proteins have very high affinity for O2 and are found in monocotyledons and legumes. LegH has attracted great interest in the vegetable meat industry owing to its organoleptic and nutritional properties. In this study, soybean LegHs A, C1, C2 and C3 and nsHb were produced via Escherichia coli-based cell-free systems (CFS) and their amino acid sequences were correctly synthesized. In addition, certain post-translational modifications were made, which were confirmed using liquid chromatography-mass spectrometry analysis. All LegHs produced in this system exhibited peroxidase activity and heme binding, which were correlated with their concentrations in the assays. Furthermore, all proteins were readily digested by pepsin within 1 min under analog digestion conditions. Thus, LegHs and nsHb proteins were produced in this study using cell-free systems, maintaining their functionality and digestibility. These findings suggest that they could serve as viable alternative food additives for plant-based meat.
Keywords: cell-free system; hemoglobins; leghemoglobins; nonsymbiotic hemoglobin; plant-based meat; protein production.
Figures


References
-
- Appleby C. A.. Leghemoglobin and rhizobium respiration. Annu. Rev. Plant Biol. 1984;35:443–478. doi: 10.1146/annurev.pp.35.060184.002303. - DOI
-
- Riquelme A., Hinrichsen P.. Non-symbiotic hemoglobin and its relation with hypoxic stress. Chil. J. Agric. Res. 2015;75(August):80–89. doi: 10.4067/S0718-58392015000300009. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

