Long-term survival of Babesia microti and Borrelia burgdorferi in C3H/HeJ mice and their effect on Lyme arthritis and babesiosis manifestations
- PMID: 40793757
- PMCID: PMC12403850
- DOI: 10.1128/spectrum.00252-25
Long-term survival of Babesia microti and Borrelia burgdorferi in C3H/HeJ mice and their effect on Lyme arthritis and babesiosis manifestations
Abstract
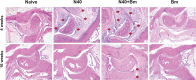
Babesia microti is increasingly co-transmitted with Lyme disease-causing Borrelia burgdorferi by ticks in the endemic regions of the United States. Infection in mice by this parasite mirrors human babesiosis, such as anemia, splenomegaly, alterations in splenic leukocyte balance, and diminished humoral immunity associated with enhanced Lyme disease manifestations at the acute phase. To evaluate the long-term survival of B. burgdorferi and B. microti in mice and their effects on pathogenesis, we conducted a 16-week infection experiment in C3H/HeJ mice. All mice infected with B. microti, irrespective of the co-infection, displayed a low-level, albeit microscopically detectable, parasitemia in both male and female mice even after 16 weeks post-infection. Splenomegaly was detected at this stage in both male and female mice and was significantly higher in females infected solely with B. microti compared to co-infected mice, likely due to a greater peak parasitemia at the acute phase of infection and persistent splenic manifestations in these mice. Interestingly, B. microti disrupted the blood-brain barrier (BBB) in mice, as reported during cerebral malaria caused by Plasmodium falciparum. Furthermore, B. burgdorferi colonization could be detected until 16 weeks of infection, while more pronounced Lyme inflammatory arthritis was observed at 4 weeks post-infection. This study underscores the complex interactions between B. microti and B. burgdorferi to affect each disease, highlighting the potential implications for vaccine development against Lyme spirochetes and therapeutic management of co-infected individuals.IMPORTANCETick-borne co-infections are becoming increasingly prevalent worldwide due to simultaneous or sequential acquisition and transmission by Ixodes species ticks during their blood meal. We reported that B. burgdorferi and B. microti co-infection reciprocally affects these pathogens during the acute phase of infection; however, the effect of co-infections on microbial long-term persistence in the murine model was not previously investigated. In this study, we have filled a critical lacuna in understanding the interactions between these two pathogens at different stages of infection and their effects on the host and disease manifestations in mice. Our investigation provides insights into their pathogenicity to allow the development of effective vaccines and successful antimicrobials against these tick-borne co-infections.
Keywords: Apicomplexan protozoan; Babesia microti; Borrelia burgdorferi; Lyme arthritis; co-infection; spirochete.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Centers for Disease Control and Prevention (CDC) . 2022. Tickborne disease surveillance data summary. Available from: https://www.cdc.gov/ticks/data-summary/index.html. Retrieved 15 Apr 2024.
-
- Centers for Disease Control and Prevention (CDC) . 2023. Surveillance for babesiosis — United States, 2020 annual summary. Available from: https://www.cdc.gov/parasites/babesiosis/resources/Surveillance_Babesios.... Retrieved 15 Apr 2024.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

