The Role of Lactate in Mitochondrial Metabolism of DOX-Induced Senescent AC16 Cells
- PMID: 40794101
- PMCID: PMC12341654
- DOI: 10.1002/cbf.70110
The Role of Lactate in Mitochondrial Metabolism of DOX-Induced Senescent AC16 Cells
Abstract
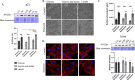
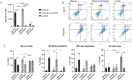
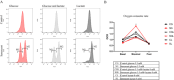
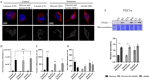
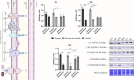
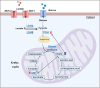
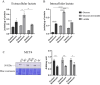
Senescent cells accumulate with age in organ and tissue causing the decline of functionality and various pathological conditions including cardiovascular disease. Regular exercise induces continuous exposure to lactate that contribute to adaptive process through mitochondrial biogenesis and improve of metabolic process. Lactate accumulation during exercise also appears to be associated with exercise-induced mitochondrial adaptation. Improvement of mitochondria function through lactate exposure could be a tool to prevent cardiomyocytes senescence and cardiac aging. The aim of the following article is to investigate the role of lactate in Doxorubicin-induced senescent AC16 human cardiomyocytes cell mitochondrial metabolism. We assessed the metabolic behaviour in senescent cardiomyocytes after chronic lactate exposure and provided a discussion of the effect of this metabolite in regulating mitochondrial physiology during cardiac aging.
Keywords: DOX‐induced senescent AC16 cells; metabolomics; mitochondrial dysfunction; proteomics.
© 2025 The Author(s). Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

