Transcriptome profiling of L. infantum-infected human macrophages reveals sex-specific type I interferon induction
- PMID: 40794782
- PMCID: PMC12367141
- DOI: 10.1371/journal.ppat.1013427
Transcriptome profiling of L. infantum-infected human macrophages reveals sex-specific type I interferon induction
Abstract
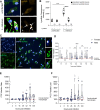
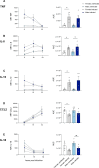
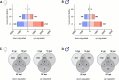
Sex-based differences in the immune system influence the clinical course of infectious diseases, including many parasitic infections. Field studies of human infections and controlled experimental rodent models have shown that certain clinical forms of leishmaniasis occur more frequently in males. Leishmania parasites infect and proliferate in innate immune cells, particularly macrophages, and modulate early immune responses that constrain their survival and replication. In this study, we used a high-throughput in vitro system to assess sex differences in human macrophage-specific immunity to Leishmania (L.) infantum infection. Quantification of infection showed significantly higher infection rates and parasite loads in macrophages derived from men compared to those from women up to 76 hours post-infection (hpi). Evaluation of the macrophage phenotype during L. infantum infection revealed only minor changes in the proportions of primarily proinflammatory M1-like macrophages, whereas a reduction in the anti-inflammatory M2-like phenotype was observed in both sexes. Cytokine profiling revealed elevated levels of TNF, IL-8, IL-10, and reduced levels of IL-18 and CCL2 in culture supernatants over the time of infection. Transcriptomic analysis showed the highest adaptation of gene expression at 6 hpi, which was more pronounced in female-derived macrophages (1428 down-regulated/2145 up-regulated genes) compared to male-derived macrophages (972 down-regulated/1637 up-regulated genes), and gradually decreased over time in both sexes. Genes associated with type I interferon responses (e.g., IFIT2, IFIT3, IFIT5, OASL, JAK1), specific cytokine response (IL-15, IL-1R1), and the matrix metalloproteinase MMP9 were up-regulated in female macrophages, while genes encoding proinflammatory chemokines involved in immune cell recruitment (CXCL1, CXCL3, CCL20, CCL7) were up-regulated in male macrophages. Treatment of infected macrophages with estradiol conferred marginal resistance to infection in female-derived macrophages, whereas testosterone treatment had no effect. In summary, our findings reveal immune mediators and underscore a biological sex difference that may explain females' superior ability to combat Leishmania infections.
Copyright: © 2025 Bea et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

