Sensitive Detection of Specific Volatile Organic Compounds by Functionalized Transition Metal Dichalcogenide Monolayers
- PMID: 40795109
- PMCID: PMC12392728
- DOI: 10.1021/acs.langmuir.5c02852
Sensitive Detection of Specific Volatile Organic Compounds by Functionalized Transition Metal Dichalcogenide Monolayers
Abstract
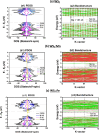
Timely detection of liver cirrhosis (LC) is critical for effective clinical management and improved patient outcomes. Among emerging diagnostic approaches, detection of volatile organic compounds (VOCs), related to LC, offers a noninvasive, rapid, and cost-effective alternative to conventional methods. In this work, we employed spin-polarized density functional theory (DFT) to systematically investigate the interaction of LC-related VOCs using transition-metal dichalcogenides (TMDs), specifically WX2 monolayers (X = S, Se2). Five VOCs, namely, 2-pentanone, dimethyl sulfide (DMS), isoprene, limonene, and methanol, were selected based on their experimental association with LC. To enhance the sensitivity and selectivity of TMDs, Mn and Fe atoms were used to dope the chalcogen sites of WX2, inducing strong dipole moments and improved van der Waals (vdW) interactions. The doped systems demonstrated significantly higher adsorption energies (Eads, 1.5-2.1 eV), charge transfer (Δq = 0.4-0.8 e), and magnetization changes (ΔM ≠ 0) for VOCs compared to air molecules (Eads < 0.5 eV, Δq < 0.1 e, ΔM = 0), confirming strong selectivity. Work function shifts Δϕ > 0.4 eV (for VOCs) and changes in the density of states near the Fermi level further support enhanced electronic response upon VOC adsorption. Our study offers atomic-scale insights into adsorption energetics, charge transfer, and electronic structure modulation that can guide future experimental efforts in nanobiosensor development. We also critically examine the scope and limitations of our theoretical framework, emphasizing the need for experimental validation to translate these findings into practical diagnostic technologies.
Figures











References
-
- World Health Organization. Global Burden of Disease: Liver Cirrhosis Mortality Statistics. In WHO Reports, 2020.
-
- American Liver Foundation. Liver Disease Statistics, 2021.
LinkOut - more resources
Full Text Sources

