Risk of Death and Adverse Effects in Patients on Liothyronine: A Multisource Systematic Review and Meta-analysis
- PMID: 40795305
- PMCID: PMC12527464
- DOI: 10.1210/clinem/dgaf449
Risk of Death and Adverse Effects in Patients on Liothyronine: A Multisource Systematic Review and Meta-analysis
Abstract
Context: Although some patients with hypothyroidism prefer combination therapy with liothyronine (LT3) and levothyroxine (LT4), the safety of LT3 remains unresolved.
Objective: We undertook a multisource systematic review and meta-analysis of LT3 safety.
Data sources: We searched PubMed for articles relating to death, adverse events (AEs), and cardiovascular outcomes in LT3 users. We also searched AEs data in the UK Yellow Card scheme and US Food and Drug Administration Adverse Reporting System (FAERS).
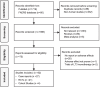
Data extraction: Data was extracted independently by 2 reviewers. Out of 1814 articles identified, 52 studies were selected, comprising 21 randomized controlled trials (RCTs), 4 cohort studies, and 27 case reports. Meta-analyses were conducted for adverse outcomes in RCTs and cohort studies of combination vs monotherapy.
Data synthesis: LT3-related AEs were only reported with unregulated LT3 use or pharmacy compounding errors. LT3 and LT4 showed similar adverse severity profiles in the Yellow Card scheme. Disproportionality analysis in the FAERS database showed no increased LT3 safety signals. A meta-analysis of RCTs (n = 2128) showed a similar AEs risk for combination vs monotherapy [relative risk (RR) 1.22, 95% confidence interval (CI) 0.66-2.25]. A cohort study meta-analysis (LT3 vs LT4-only users, n = 630 254) showed no increased risk of atrial fibrillation (RR 1.10, 95% CI 0.74-1.63), heart failure (RR 1.54, 95% CI 0.95-2.47), or strokes (RR 0.86, 95% CI 0.11-6.75), but reduced mortality risk was observed for LT3 (RR 0.70, 95% CI 0.62-0.78).
Conclusion: Our findings are reassuring that regulated LT3 use is not associated with the risk of death or serious AEs. More studies are needed to supplement existing data.
Keywords: adverse events; cardiovascular; death; hypothyroidism; levothyroxine; liothyronine.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Comment in
-
Safe optimization of thyroid hormone therapy.J Clin Endocrinol Metab. 2025 Sep 10:dgaf512. doi: 10.1210/clinem/dgaf512. Online ahead of print. J Clin Endocrinol Metab. 2025. PMID: 40929373 No abstract available.
References
-
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301‐316. - PubMed
-
- Taylor PN, Medici MM, Hubalewska-Dydejczyk A, Boelaert K. Hypothyroidism. Lancet. 2024;404(10460):1347‐1364. - PubMed
-
- Okosieme OE. Thyroid hormone replacement: current status and challenges. Expert Opin Pharmacother. 2011;12(15):2315‐2328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical