Body mass index and subsequent fracture risk: a meta-analysis to update FRAX
- PMID: 40795319
- PMCID: PMC12487781
- DOI: 10.1093/jbmr/zjaf091
Body mass index and subsequent fracture risk: a meta-analysis to update FRAX
Abstract
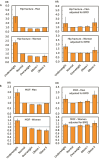
The aim of this international meta-analysis was to quantify the predictive value of BMI for incident fracture and relationship of this risk with age, sex, follow-up time, and BMD. A total of 1 667 922 men and women from 32 countries (63 cohorts), followed for a total of 16.0 million person-years were studied. 293 325 had FN BMD measured (2.2 million person-years follow-up). An extended Poisson model in each cohort was used to investigate relationships between WHO-defined BMI categories (Underweight: <18.5 kg/m2; Normal: 18.5-24.9 kg/m2; Overweight: 25.0-29.9 kg/m2; Obese I: 30.0-34.9 kg/m2; Obese II: ≥35.0 kg/m2) and risk of incident osteoporotic, major osteoporotic and hip fracture (HF). Inverse-variance weighted β-coefficients were used to merge the cohort-specific results. For the subset with BMD available, in models adjusted for age and follow-up time, the hazard ratio (95% CI) for HF comparing underweight with normal weight was 2.35 (2.10-2.60) in women and for men was 2.45 (1.90-3.17). Hip fracture risk was lower in overweight and obese categories compared to normal weight [obese II vs normal: women 0.66 (0.55-0.80); men 0.91 (0.66-1.26)]. Further adjustment for FN BMD T-score attenuated the increased risk associated with underweight [underweight vs normal: women 1.69 (1.47-1.96); men 1.46 (1.00-2.13)]. In these models, the protective effects of overweight and obesity were attenuated, and in both sexes, the direction of association reversed to higher fracture risk in Obese II category [Obese II vs Normal: women 1.24 (0.97-1.58); men 1.70 (1.06-2.75)]. Results were similar for other fracture outcomes. Underweight is a risk factor for fracture in both men and women regardless of adjustment for BMD. However, while overweight/obesity appeared protective in base models, they became risk factors after additional adjustment for FN BMD, particularly in the Obese II category. This effect in the highest BMI categories was of greater magnitude in men than women. These results will inform the second iteration of FRAX®.
Keywords: BMI; FRAX; epidemiology; hip fracture; major osteoporotic fracture; meta-analysis; osteoporosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
N.C.H. has received consultancy/lecture fees/honoraria/grant funding from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Radius Health, Servier, Shire, UCB, Consilient Healthcare, Kyowa Kirin, Theramex, and Internis Pharma.
J.A.K. led the team that developed FRAX as director of the WHO Collaborating Centre for Metabolic Bone Diseases. E.V.M., W.D.L., M.L., N.C.H., E.L., L.V., and H.J. are members of the FRAX team. J.A.K., N.C.H., and E.V.M. are members of the advisory body to the National Osteoporosis Guideline Group. J.A.K. reports no additional competing interests.
K.E.Å. has no financial interest related to FRAX; chaired the National SALAR Group for Person-Centered Care Pathway Osteoporosis.
F.A.A. led the team that developed GLOW, while director of the Center for Outcomes Research at the University of Massachusetts Medical School; he has no financial interest in FRAX.
R.A. has received funding for research from Instituto Carlos III of Spanish Ministry of Health, IDIAP Jordi Gol of Catalan Government and from Scientific Societies SEMFYC and SEIOMM.
C.L.B. is employed at Nordic Bioscience and owns stock in Nordic Bioscience. She declares no competing interests in relation to this work.
H.A.B.-F. has no financial interest in FRAX. For the DO-HEALTH trial cohort, Prof. H.A.B.-F. reports independent and investigator-initiated grants from European Commission Framework 7 Research Program, from the University of Zurich, from NESTEC, from Pfizer Consumer Healthcare, from Streuli Pharma, plus non-financial support from DNP. For the study cohort extension, she reports independent and investigator-initiated grants from Pfizer and from Vifor. Further, Prof. H.A.B.-F. reports non-financial support from Roche Diagnostics and personal fees from Wild, Sandoz, Pfizer, Vifor, Mylan, Roche, Meda Pharma, outside the submitted work with regard to speaker fees and travel fees.
J.R.C. has received honoraria for speaking at educational meetings and for advisory boards from Amgen and honoraria for an advisory board from Bayer, all unrelated to this work. R.C. has no financial interest in FRAX. He has received grant funding from Amgen, UCB, Chugai, MSD, Mylan, and Medac. He has received honoraria from Amgen, UCB, Chugai, Galapagos, Biocon, Abbvie, Haoma Medica, Pfizer, Amolyt, MSD, Lilly, BMS, Novartis, Arrow, PKMed, Kyowa-Kirin, and Sanofi.
C.C. owns stock in Nordic Bioscience. He declares no competing interests in relation to this work.
C.C. reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB.
A.D.-P. reports personal fees from Amgen, Lilly, Theramex, and grants from Instituto Carlos III and owns shares of Active Life Scientific, all outside the submitted work.
J.A.E. declares consulting and research support from Actavis, Amgen, Aspen, Lilly, Merck Sharp and Dohme, Novartis, Sanofi-Aventis, Servier, and Theramex.
P.J.M.E. has no financial interest in FRAX. P.J.M.E. reports support for the SOS study by Stichting Achmea Gezondheidszorg, Achmea, and VGZ zorgverzekeraar. Additional support was given by the stichting Artsenlaboratorium en Trombosedienst. Outside the submitted work, she did receive independent investigator driven grants by Zonmw, The Netherlands, de Hartstichting, The Netherlands, The European foundation for the study of Diabetes, Amgen, The Netherlands, TEVA, The Netherlands, and Takeda, The Netherlands.
C.-C.G. reports honoraria and research support from AgNovos, Amgen, osteolabs, and UCB unrelated to this work.
D.P.K. has no financial interest in FRAX but has received support for his work in the Framingham Study over the past 30 yr by the National Institutes of Health, Astra Zeneca, Merck, Amgen, and Radius Health.
M.A.K. has received funding from the National Health and Medical Research Council (NHMRC) Australia, and the Medical Research Future Fund (MRFF) Australia. He has served on advisory boards for Amgen Australia, Novartic, and Eli Lilly—all unrelated to this work and was the Director of the Geelong Bone Densitometry Service until 2022.
A.L. is PI of the HUNT Lung and Osteoporosis project, the project got some funding from AstraZeneca for data collection. He has received lecture or consultant fees from AstraZeneca, GSK, Boehringer Ingelheim, and Diagnostica. No funding related to this study.
M.L. has received lecture fees from Amgen, Lilly, Meda, Gedeon Richter, Medison Pharma, and UCB Pharma and consulting fees from Amgen, Radius Health, UCB Pharma, Medac AB, and Parexel International, all outside the presented work.
E.V.M. has received consultancy/lecture fees/grant funding/honoraria from AgNovos, Amgen, AstraZeneca, Consilient Healthcare, Fresenius Kabi, Gilead, GSK, Hologic, Internis, Lilly, Merck, Novartis, Pfizer, Radius Health, Redx Oncology, Roche, Sanofi Aventis, UCB, ViiV, Warner Chilcott, and I3 Innovus.
C.O. is listed as a coinventor on two patent applications regarding probiotics in osteoporosis treatment.
T.W. O’N. reports honoraria from UCB unrelated to this work.
E.S.O. reports consulting fees from Angios, Biocon, Radius, and Bayer.
J.A.P. has received funding from the National Health and Medical Research Council (NHMRC) Australia, and the Medical Research Future Fund (MRFF) Australia, and Amgen, all unrelated to this work.
K.M.A.S. is an employee of the PHARMO Institute for Drug Outcomes Research. This independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies.
N.C.W. sits on the Board of Trustee of the US Bone Health and Osteoporosis Foundation, and has received consulting fees from Radius and ArgenX.
M.C.Z. reports honoraria for lectures and consulting from Amgen and Kyowa Kirin and research support from Kyowa Kirin to her institution, unrelated to this work.
M.Z. has received research funding from national societies (SEMFYC and SEIOMM).
Figures

References
-
- Wolk R, Berger P, Lennon RJ, Brilakis ES, Somers VK. Body mass index: a risk factor for unstable angina and myocardial infarction in patients with angiographically confirmed coronary artery disease. Circulation. 2003;108(18):2206–2211. - PubMed
-
- Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. - PubMed
-
- Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27(1):1–10. - PubMed
-
- Neri SGR, Oliveira JS, Dario AB, Lima RM, Tiedemann A. Does obesity increase the risk and severity of falls in people aged 60 years and older? A systematic review and meta-analysis of observational studies. J Gerontol A Biol Sci Med Sci. 2020;75(5):952–960. - PubMed
Publication types
MeSH terms
Grants and funding
- 75N92021D00002/HL/NHLBI NIH HHS/United States
- 75N92021D00005/WH/WHI NIH HHS/United States
- HHSN268201500001C/HL/NHLBI NIH HHS/United States
- R01 AR035583/AR/NIAMS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- R01 AR035584/AR/NIAMS NIH HHS/United States
- U01 AG042145/AG/NIA NIH HHS/United States
- 75N92021D00001/HL/NHLBI NIH HHS/United States
- R01 AG005407/AG/NIA NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- U01 AG042168/AG/NIA NIH HHS/United States
- U01 AG042140/AG/NIA NIH HHS/United States
- R01 AR035582/AR/NIAMS NIH HHS/United States
- U01 AG042139/AG/NIA NIH HHS/United States
- U01 AR066160/AR/NIAMS NIH HHS/United States
- R01 AG066671/AG/NIA NIH HHS/United States
- U01 AG042124/AG/NIA NIH HHS/United States
- R01 AR061445/AR/NIAMS NIH HHS/United States
- R01 AR041398/AR/NIAMS NIH HHS/United States
- HHSN268201500001I/HL/NHLBI NIH HHS/United States
- 75N92021D00003/WH/WHI NIH HHS/United States
- U01 AG027810/AG/NIA NIH HHS/United States
- R01 AG005394/AG/NIA NIH HHS/United States
- 75N92021D00004/WH/WHI NIH HHS/United States
- U01 AG042143/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

