New molecular diagnostic targets for Avibacterium paragallinarum and a set of single-plex and multiplex qPCR methods for the rapid differential diagnosis of Mycoplasma gallisepticum, Mycoplasma synoviae, and Avibacterium paragallinarum
- PMID: 40795612
- PMCID: PMC12361822
- DOI: 10.1016/j.psj.2025.105665
New molecular diagnostic targets for Avibacterium paragallinarum and a set of single-plex and multiplex qPCR methods for the rapid differential diagnosis of Mycoplasma gallisepticum, Mycoplasma synoviae, and Avibacterium paragallinarum
Abstract
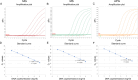
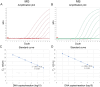
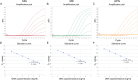
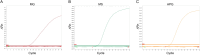
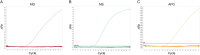
Mycoplasma gallisepticum (MG), Mycoplasma synoviae (MS), and Avibacterium paragallinarum (APG) are respiratory-borne bacterial pathogens that severely harm the poultry industry. The clinical symptoms caused by them share many similarities, such as respiratory disease, growth retardation, and decreased egg production. They are not suitable for rapid diagnosis through isolation and culture and often need to detect nucleic acids or antibodies for differential diagnosis. In this study, bioinformatics analyses were used, and six specific coding genes were identified as being shared among all the APG strains that were absent in other species with published genome sequences. Combined with MG- and MS-specific genes identified in previous studies, we established a set of single-plex and multiplex qPCR assays for the rapid differential diagnosis of these three pathogens. The results indicated that the correlation coefficients (R2) of the standard curve established in these methods were not less than 0.999, and the amplification efficiencies (E) were between 90 % and 110 %. In terms of specificity, with the exception of the amplification curve and CT value generated in the positive control, other related pathogens, chicken cells, and empty plasmid did not amplify. In terms of sensitivity, the 100 % detection sensitivity of MG single-plex qPCR, MS single-plex qPCR, APG single-plex qPCR, and MG-MS duplex qPCR established in this study was 5 copies/reaction. The 100 % detection sensitivity of MG-MS-APG triplex qPCR was 5 copies/reaction in both the MG and MS detection channels and 10 copies/reaction in the APG detection channel. The detection rate of triplex qPCR in the APG detection channel at 5 copies/reaction was 80 %. The intra-group and inter-group variation coefficients of the qPCR methods established in this study were all within 2 % in the repeatability evaluation. In terms of the coincidence rate of clinical sample testing, the qPCR methods showed 100 % detection consistency for the clinical samples tested. The established qPCR methods exhibited good specificity, sensitivity, and repeatability, which provide powerful technical support for the rapid and efficient differential diagnosis of MG, MS, and APG simultaneously.
Keywords: Avibacterium paragallinarum; Mycoplasma gallisepticum; Mycoplasma synoviae; diagnostic target; molecular diagnosis.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Disclosures The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
The quadruplex fluorescent quantitative PCR method for the simultaneous detection of respiratory diseases in quail: Pasteurella multocida, Avibacterium paragallinarum, Mycoplasma gallisepticum, and Mycoplasma synoviae.Front Microbiol. 2025 Jun 30;16:1605356. doi: 10.3389/fmicb.2025.1605356. eCollection 2025. Front Microbiol. 2025. PMID: 40661987 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Development and Validation of PCR Assays for Improved Diagnosis of Infectious Coryza by Differentiating Pathogenic and Nonpathogenic Avibacterium paragallinarum.Avian Dis. 2025 Apr;68(S1):380-390. doi: 10.1637/aviandiseases-D-24-00041. Avian Dis. 2025. PMID: 40249576
-
Pooled molecular occurrence of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry: A systematic review and meta-analysis.Transbound Emerg Dis. 2022 Sep;69(5):2499-2511. doi: 10.1111/tbed.14302. Epub 2021 Aug 30. Transbound Emerg Dis. 2022. PMID: 34427387
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Alajaji A.I. A review of the advanced immunogens for the protection of poultry flocks against infectious laryngotracheitis virus. Pak. Vet. J. 2024;44:1006–1012.
-
- Amorim M.M.R., Bandeira R.P., Clemente S.M., Vilela S., Mota R.A., Nascimento E.R., Barros M.R. Serological and molecular diagnosis of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry farms. Braz. J. Poult. Sci. 2024;26
-
- Anggriawan R., Lokapirnasari W.P., Hidanah S., Al Arif M.A., Candra D.A. Residue detection of tylosin antibiotics and enrofloxacin in broiler chickens. Adv. Anim. Vet. Sci. 2024;12:2195–2204.
-
- Babazadeh D., Abd El-Ghany W.A. Distribution, infection, diagnosis, and control of avibacterium paragallinarum in poultry. Kafkas. Univ. Vet. Fak. Derg. 2023;29:595–609.
-
- Bashashati M., Banani M. Complete sequence-based genotyping of mgc2/pvpA genes in chicken-derived Mycoplasma gallisepticum isolates of Iran. Avian Dis. 2020;64:507–516. - PubMed
LinkOut - more resources
Full Text Sources

