Exploratory subgroup analyses of EV-302: a phase III global study to evaluate enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced or metastatic urothelial carcinoma
- PMID: 40795788
- PMCID: PMC12361773
- DOI: 10.1016/j.esmoop.2025.105544
Exploratory subgroup analyses of EV-302: a phase III global study to evaluate enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced or metastatic urothelial carcinoma
Abstract
Background: In the phase III EV-302 study (NCT04223856), enfortumab vedotin (EV) plus pembrolizumab (P) demonstrated superior efficacy and safety versus platinum-based chemotherapy in patients with previously untreated locally advanced/metastatic urothelial cancer (la/mUC). We report the efficacy of EV+P in prespecified subgroups, including those defined by cisplatin eligibility status, the presence or absence of liver metastases, and metastatic disease sites.
Methods: Patients with previously untreated la/mUC were randomly assigned 1 : 1 to receive either EV 1.25 mg/kg and pembrolizumab 200 mg, or gemcitabine plus cisplatin or carboplatin, all intravenously. The two primary endpoints were progression-free survival (PFS) and overall survival (OS). Confirmed objective response rate was one of the secondary endpoints.
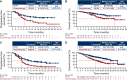
Results: Overall, 886 patients were randomized: 442 to EV+P and 444 to chemotherapy. Baseline characteristics were balanced across treatment groups. Efficacy and safety data for the intention-to-treat (ITT) population, along with PFS and OS data for cisplatin-eligible and -ineligible patients, were previously published (Powles et al. N Eng J Med, 2024). In this analysis, EV+P showed benefit across prespecified subgroups that was consistent with the ITT population. OS benefit in the EV+P arm versus chemotherapy was seen across all subgroups, including patients with liver metastases (OS 19.1 versus 10.1 months), patients without liver metastases [OS not estimable (NE) versus 17.9 months], patients with visceral metastases (OS 25.6 versus 13.6 months), and in patients with lymph node-only disease (OS NE versus 27.5 months). In addition, confirmed objective response rate and PFS benefit with EV+P versus chemotherapy was seen across all examined subgroups.
Conclusion: Along with previously published safety data, EV+P demonstrated benefit compared with chemotherapy across all prespecified subgroups, consistent with the ITT population and supporting EV+P as the standard of care for first-line treatment of la/mUC.
Keywords: bladder cancer; enfortumab vedotin; metastatic urothelial carcinoma; pembrolizumab; phase III; subgroup analysis.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- National Cancer Institute Cancer stat facts: bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html Available at.
-
- Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

