Allografts in primary anterior cruciate ligament reconstruction : a scoping review of the literature highlighting reporting standards
- PMID: 40796153
- PMCID: PMC12343146
- DOI: 10.1302/2633-1462.68.BJO-2025-0080.R1
Allografts in primary anterior cruciate ligament reconstruction : a scoping review of the literature highlighting reporting standards
Erratum in
-
Corrigendum.Bone Jt Open. 2025 Nov 12;6(11):1456. doi: 10.1302/2633-1462.611.BJO-2025-00006. Bone Jt Open. 2025. PMID: 41218650 Free PMC article. No abstract available.
Abstract
Aims: To conduct a scoping review into the use of allograft in primary anterior cruciate ligament (ACL) reconstruction, and to ascertain the variability in reporting outcomes in the literature.
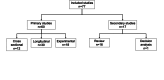
Methods: The study was conducted in line with the Preferred Reporting Items for Systematic reviews and Meta-Analayses (PRISMA), and also used Arksey and O'Malley's established five-stage process for scoping reviews in order to map the literature for allograft use in primary ACL reconstruction. Following screening to identify eligible studies, data were extracted and mapped to provide a descriptive and thematic analysis.
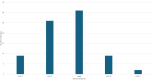
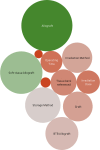
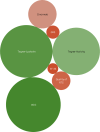
Results: A total of 421 studies were identified from the initial search, with 77 studies eligible for final scoping review published from January 1993 to December 2024. The majority of studies were published from the USA and China (56/77, 72.3%). Nine studies (9/77, 11.7%) were level1 evidence. Key variables such as graft diameter (27/77, 33.8%), graft processing (27/77, 35.1%), and cost of graft (3/77, 3.9%) were significantly under-reported. For clinical outcomes, the Lachman score (45/77, 57.1%), pivot shift grade (45/77, 58.4%), and graft re-rupture rate (42/77, 54.5%) were highest reported. For functional outcomes, two predominant scores were recorded, the International Knee Documentation Committee score (52/77, 67.5%) and the Tegner-Lysholm knee score (48/77, 62.3%). A total of 30 functional outcomes were recorded, spanning all studies.
Conclusion: This scoping review identified 77 studies which analyzed allografts in primary ACL reconstruction. There is great variability in the reporting standards, with significant under-reporting of important variables. Further research is required to develop standardized reporting criteria in order to accurately reflect the outcomes of allografts in primary ACL reconstruction.
© 2025 Al-Hourani et al.
Conflict of interest statement
K. Al-Hourani reports consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Meril Life, unrelated to this study. F. S. Haddad reports multiple research study grants from Stryker, Smith & Nephew, Corin, International Olympic Committee, and the NIHR, royalties or licenses from Smith & Nephew, Stryker, Corin, and MatOrtho, consulting fees from Stryker, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Stryker, Smith & Nephew, Zimmer, AO Recon, and Mathys, and support for attending meetings and/or travel from Stryker, Mathys, AO Recon, and The Bone & Joint Journal, all of which are unrelated to this study. F. S. Haddad is also Editor in Chief of The Bone & Joint Journal, President of the International Hip Society, Vice President of the European Hip Society, and an ISTA board member. S. Khan is a surgical advisor for Orthonika Limited. X. Li is a consultant for FH Ortho. I. R. Murray reports consulting fees from Stryker, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Arthrex and Smith & Nephew, all of which are unrelated to this study.
Figures


References
-
- Goetz G, de Villiers C, Sadoghi P, Geiger-Gritsch S. Allograft for anterior cruciate ligament reconstruction (ACLR): a systematic review and meta-analysis of long-term comparative effectiveness and safety. Results of a health technology assessment. Arthrosc Sports Med Rehabil. 2020;2(6):e873–e891. doi: 10.1016/j.asmr.2020.07.003. - DOI - PMC - PubMed
-
- Gabr A, Haddad FS, NJR Steering Committee . The National Ligament Registry; 2022. [4 August 2025]. The Seventh Annual Report.https://www.uknlr.co.uk/pdf/annual-report-2022.pdf date last. accessed. - DOI
LinkOut - more resources
Full Text Sources

