MammaPrint predicts chemotherapy benefit in HR+HER2- early breast cancer: FLEX Registry real-world data
- PMID: 40796181
- PMCID: PMC12413225
- DOI: 10.1093/jncics/pkaf079
MammaPrint predicts chemotherapy benefit in HR+HER2- early breast cancer: FLEX Registry real-world data
Abstract
Background: Gene expression assays help personalize adjuvant chemotherapy decisions for hormone receptor-positive, HER2-negative (HR+HER2-) early breast cancer (EBC). The 70-gene risk of distant-recurrence signature, MammaPrint, demonstrated clinical utility in guiding chemotherapy de-escalation in genomically low risk patients in the MINDACT trial. This study evaluates MammaPrint as a continuous predictor of chemotherapy benefit in HR+HER2- EBC using real-world data (RWD) from the FLEX Registry.
Methods: The study evaluated 1002 patients treated with endocrine therapy (ET) only or ET with chemotherapy (ET+CT) enrolled in FLEX (NCT03053193) with 5-year median follow-up. Propensity-score matching balanced treatment groups by menopausal status, T-stage, and nodal status. The primary endpoint was distant recurrence-free interval (DRFI). Regression and Cox proportional hazards models assessed chemotherapy benefit across MammaPrint Index (MPI) risk.
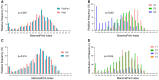
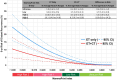
Results: Most patients were postmenopausal (70.1%), node-negative (70.0%), and had grade 2 tumors (51.2%). The regression models showed that MPI strongly predicted 5-year DRFI in ET only (R2 = 0.99, P < .001) and ET + CT (R2 = 0.90, P < .001) groups, corresponding to an average absolute chemotherapy benefit of 5.6% in High 1 and 10.9% in High 2. Minimal improvement in DRFI with chemotherapy was observed for Low (1.7%) and UltraLow (<1.0%) risk groups. A multivariate Cox model with an MPI-by-treatment interaction term demonstrated that increasing MPI risk was associated with greater chemotherapy benefit on DRFI (HR = 0.15, P = .047). Chemotherapy benefit was significantly associated with premenopausal status, but not age, T-stage, nodal status, or grade.
Conclusions: These RWD from the FLEX Registry demonstrate that MPI is predictive of both DRFI prognosis and chemotherapy benefit in HR+HER2- EBC. (NCT03053193).
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
The authors have the following paid financial relationships with the funder:
Receive research funding from funder: Adam Brufsky, Kent Hoskins, Henry Conter, Pond Kelemen, Mehran Habibi, Laila Samian, Rakshanda Rahman, Laura Lee, Eduardo Dias, Regina Hampton, Beth Sieling, Cynthia Osborne, Eric Brown, Jailan Elayoubi, Priyanka Sharma, Jayanthi Ramadurai, Laurie Matt-Amaral, Alfredo Santillan, Sasha Strain, Philip Albaneze, Pat Whitworth, Nathalie Johnson, and Joyce O’Shaughnessy.
Speakers for funder: Adam Brufsky, Henry Conter, Eric Brown, Beth Sieling, Alfredo Santillan.
Consultants for funder: Adam Brufsky and Joyce O’Shaughnessy.
Employed and have stock ownership in funding company: Harshini Ramaswamy, Nicole Chmielewski-Stivers, Andrea Menicucci, and William Audeh.
Figures

Comment in
-
Leveraging real-world evidence to personalize adjuvant therapy in HR+/HER2- early breast cancer.JNCI Cancer Spectr. 2025 Sep 1;9(5):pkaf092. doi: 10.1093/jncics/pkaf092. JNCI Cancer Spectr. 2025. PMID: 41143549 Free PMC article. No abstract available.
References
-
- SEER Cancer Stat Facts: Female Breast Cancer Subtypes. National Cancer Institute. Accessed January 9, 2025. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
-
- Rashmi Kumar N, Schonfeld R, Gradishar WJ, et al. NCCN guidelines version 6.2024 breast cancer. 2024. https://www.nccn.org/
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
