Effectiveness of screening and ultra-brief intervention for hazardous drinking in primary care: pragmatic cluster randomised controlled trial
- PMID: 40796231
- PMCID: PMC12340667
- DOI: 10.1136/bmj-2024-083985
Effectiveness of screening and ultra-brief intervention for hazardous drinking in primary care: pragmatic cluster randomised controlled trial
Abstract
Objective: To evaluate the effectiveness of a doctor delivered screening and ultra-brief intervention (<1 minute) compared with simplified assessment only for reducing alcohol intake among patients with hazardous drinking in primary care.
Design: Pragmatic cluster randomised controlled trial.
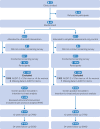
Setting: 40 primary care clinics in Japan that did not provide routine screening and brief intervention for hazardous drinking or treatment or self-help groups for alcohol dependency.
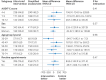
Participants: 1133 outpatients aged 20-74 years with hazardous drinking (AUDIT-C (alcohol use disorders identification test-consumption) scores ≥5 for men and ≥4 for women). Clinic clusters were allocated to a study arm using a computer generated random sequence. Participants and staff who collected participant reported outcomes remained blinded to assignment.
Interventions: Primary care clinics were randomised to ultra-brief intervention (21 clinics, 531 patients) or simplified assessment only (19 clinics, 602 patients) groups. The intervention group comprised screening with AUDIT-C followed by brief oral advice and an alcohol information leaflet delivered in <1 minute. The control group comprised simplified assessment with AUDIT-C only.
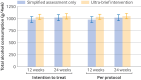
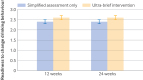
Main outcome measures: The primary outcome was total alcohol consumption in the four weeks preceding the 24 week follow-up. Secondary outcomes included total alcohol consumption in the four weeks preceding the 12 week follow-up, and readiness to change drinking behaviour, measured at 12 and 24 weeks.
Results: At 24 weeks, the difference in total alcohol consumption between the ultra-brief intervention group (1046.9 g/4 weeks (g/4wk), 95% confidence interval (CI) 918.3 to 1175.4) and control group (1019.0 g/4wk, 893.5 to 1144.6) was 27.8 g/4wk (-149.7 to 205.4, P=0.75), with a Hedges' g of 0.02 (95% CI -0.10 to 0.14). At 12 weeks, the difference in total alcohol consumption between the intervention group (1034.1 g/4wk, 919.6 to 1148.7) and control group (979.3 g/4wk, 866.1 to 1092.4) was 54.9 g/4wk (-104.1 to 213.9, P=0.49), with a Hedges' g of 0.04 (-0.08 to 0.16).
Conclusion: This trial found no evidence to support the effectiveness of a doctor delivered ultra-brief intervention for hazardous drinking compared with simplified assessment only in primary care in Japan.
Trial registration: UMIN Clinical Trials Registry UMIN000051388.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Japan Agency for Medical Research and Development (AMED) awarded to RS, KN, and SM. No other direct support was received from any organisation for the submitted work. Financial relationships with organisations that might have an interest in the submitted work in the previous three years: RS and HN were employed by CureApp during the conduct of the study. RS received personal grants from the Osake-no-Kagaku Foundation and the Mental Health Okamoto Memorial Foundation outside the submitted work. He holds multiple pending patents (JP2022049590A, US20220084673A1, JP2022178215A, JP2022070086, JP2023074128A). SM received personal fees from CureApp, Otsuka Pharmaceutical, EA Pharma, Takeda Pharmaceutical, Yoshitomi Pharmaceutical Industries, Eisai, Nippon Shinyaku, and MSD KK outside the submitted work. TAF reports personal fees from Boehringer-Ingelheim, Daiichi Sankyo, DT Axis, Micron, Shionogi, Sony, and UpToDate, and a grant from DT Axis and Shionogi, outside the submitted work; in addition, TAF has a patent (7448125) and a pending patent (2022-082495), and has licensed intellectual properties for Kokoro-app to Mitsubishi-Tanabe. YT received lecture fees from MSD KK outside the submitted work. YH and HY received personal fees from CureApp and Otsuka Pharmaceutical outside the submitted work. TY received personal fees from CureApp, Otsuka Pharmaceutical, and Nippon Shinyaku outside the submitted work. KN received personal fees from CureApp for coordinating a pivotal study outside the submitted work. No other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization, 2019.
-
- Babor TF, Higgins-Biddle JC, Saunders JB, et al. The Alcohol Use Disorders Identification Test: Guidelines for use in primary care. World Health Organization, 2001.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
