Comparative efficacy between real-world and randomized studies of palbociclib+endocrine therapy in HR-positive/HER2-negative metastatic breast cancer: systematic review and meta-analysis
- PMID: 40796258
- PMCID: PMC12413244
- DOI: 10.1093/jncics/pkaf083
Comparative efficacy between real-world and randomized studies of palbociclib+endocrine therapy in HR-positive/HER2-negative metastatic breast cancer: systematic review and meta-analysis
Abstract
Background: Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) combined with endocrine therapy are the standard-of-care for hormone receptor-positive (HR+)/HER2-negative (HER2-) metastatic breast cancer (MBC). Palbociclib, the first approved CDK4/6i, significantly improved progression-free survival (PFS) in randomized controlled trials (RCTs). However, real-world (RW) outcomes may differ due to broader patient populations. This meta-analysis evaluates the applicability of pivotal RCT findings to RW settings.
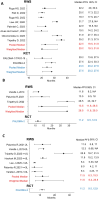
Methods: We conducted a systematic review and meta-analysis of RW studies on HR+/HER2- MBC treated with palbociclib+aromatase inhibitors (AI) or fulvestrant, reporting median PFS (mPFS) and/or overall survival (mOS). Pooled mPFS/OS was estimated using the median of medians (MM) and weighted MM (WM). RW estimates were deemed comparable to RCTs if MMPFS/OS or WMPFS/OS fell within RCTs' 95% confidence intervals (CIs). Similar criteria applied to pooled hazard ratios (HRs) of PFS/OS for palbociclib+AI vs AI in visceral/nonvisceral subgroups.
Results: Twelve RW studies were analyzed. First-line palbociclib+AI MMPFS (22.5 months, 95% CI = 19.5 to 31.8) aligned with PALOMA-1/2 pooled mPFS (23.9, 95% CI = 20.2 to 27.6). First-line palbociclib+fulvestrant MMPFS (13.5, 95% CI = 11.6 to 28.5) exceeded PALOMA-3 (11.2, 95% CI = 9.5 to 12.9). Second-line palbociclib+fulvestrant MMPFS (11.5 months, 95% CI = 6.3 to 15.3) was consistent with PALOMA-3. RW first-line mOS (51.2 months, 95% CI = 49.1 to 53.3) surpassed PALOMA-1/2 pooled mOS (45.7, 95% CI = 37.5 to 53.8). WMOS (49.1 months, 95% CI = 49.1 to 53.3) was slightly lower than RCTs (53.7, 95% CI = 37.5 to 53.8). Palbociclib+AI outperformed AI in RW visceral disease, aligning with RCTs, and showed heterogeneous but favorable benefit in nonvisceral disease.
Conclusions: RW data confirm palbociclib+endocrine therapy effectiveness, reinforcing its applicability to broader patient populations.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
B.C. reports speaker fees from Veracyte and payment for educational events from Medsite and Novartis. C.C. had consultancy/advisory role/speaker bureau for Pfizer, Roche, MSD, Novartis, Lilly, Seagen, Gilead Daiichi-Sankyo, outside the submitted work. G.C. has received research grants from Merck; has received honoraria from Ellipses Pharma; has received support for attending meetings and/or travel from Roche/Genentech, Pfizer, Daiichi Sankyo and AstraZeneca; has a leadership role for the ESMO, the European Society of Breast Cancer Specialists and ESMO Open; is a speakers’ bureau member for Roche/Genentech, Novartis, Pfizer, Lilly, Foundation Medicine, Samsung, Daiichi Sankyo, Seagen, Menarini, Gilead Sciences, AstraZeneca and Exact Sciences; and has held consulting or advisory roles for Roche/Genentech, Pfizer, Novartis, Lilly, Foundation Medicine, Bristol Myers Squibb, Samsung, AstraZeneca, Daiichi Sankyo, Boehringer Ingelheim, GlaxoSmithKline, Seagen, Guardant Health, Veracyte, Celcuity, Hengrui Therapeutics, Menarini, Merck, Exact Sciences, Blueprint Medicines, and Gilead Sciences. L.D.M. reports honoraria from Roche, Novartis, Eli Lilly, MSD, Pfizer, Ipsen, Novartis, Gilead, Olema, and Menarini Stemline; serving in an advisory role for Agendia, Roche, Eli Lilly, Novartis, MSD, Genomic Health, Pierre Fabre, Daiichi Sankyo, Seagen, AstraZeneca, Eisai, Gilead, Olema, and Menarini Stemline outside the submitted work; and travel grants from Roche, Daiichi Sankyo, AstraZeneca, and Menarini Stemline. M.C. reported consulting for AstraZeneca, Celcuity, Menarini Stemline, Repare, Olaris, Syantra, BriaCell, and Datar Genomics, receiving grants or research support from AstraZeneca and Celcuity, receiving honoraria from Pfizer, AstraZeneca, Merck, Iylon, and Menarini-Silicon Biosystem. D.G. reports personal fees for educational events by Novartis, Lilly, Pfizer, Daiichy-Sankyo, Roche; research funds from Astrazeneca, Novartis, and LILT. M.G. reported receiving personal fees from AstraZeneca, Lilly, Novartis, Pfizer, Daiichi Sankyo, MSD, Gentili, Menarini Stemline, Exact Sciences, and Eisai outside the submitted work. M.L. reports advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD, Exact Sciences, Pierre Fabre, Menarini; speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo, Takeda, Menarini, AstraZeneca; travel Grants from Gilead, Daiichi Sankyo, Roche; research funding (to the Institution) from Gilead all outside the submitted work. T.P. reports speaker’s fee from Pfizer, AstraZeneca, Novartis, Veracyte, and Argenetics; advisory role with Novartis. H.S.R. reports grant/research support from AstraZeneca, Daiichi Sankyo, Inc., F. Hoffmann-La Roche AG/Genentech, Inc., Gilead Sciences, Eli Lilly, Merck, Novartis Pharmaceuticals Corporation, Pfizer, Stemline Therapeutics, OBI Pharma, and Ambryx; and honoraria from NAPO, PUMA, Sanofi, Mylan, and Chugai. R.S.-B. reports advisory/consulting/speaker fees from Roche, AstraZeneca, Novartis, Lilly, Daiichi Sankyo, Pfizer, Eisai, GlaxoSmithKline, Reveal Genomics, and Gilead; travel expenses from Pfizer, AstraZeneca, Gilead, Novartis, and Roche. F.S. reports honoraria from Novartis, Gilead, Veracyte, and Daiichy-Sankyo for educational events/materials, advisory fees from Pfizer, Daiichy-Sankyo, and Veracyte, and travel expenses from Novartis, Gilead, and Daiichy-Sankyo. The other authors have nothing to declare.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209-249. - PubMed
-
- Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20:1360-1369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
