The sodium/glucose cotransporter 2 inhibitor empagliflozin is a pharmacological chaperone of cardiac Nav1.5 channels
- PMID: 40796268
- PMCID: PMC7618122
- DOI: 10.1152/ajpheart.00363.2025
The sodium/glucose cotransporter 2 inhibitor empagliflozin is a pharmacological chaperone of cardiac Nav1.5 channels
Abstract
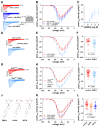
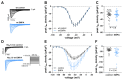
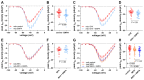
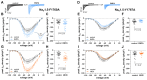
Diminished peak sodium current (INa) is a causative factor for slowed ventricular conduction and cardiac arrhythmias in patients with Duchenne muscular dystrophy (DMD), a devastating muscle disease triggered by dystrophin deficiency. Recently, we showed that chronic administration of the sodium/glucose cotransporter 2 (SGLT2) inhibitor empagliflozin (EMPA) restores diminished peak INa in ventricular cardiomyocytes from the dystrophin-deficient mdx mouse model of DMD. Here, we aimed to elucidate the underlying mechanism. Whole cell patch clamp studies revealed that 24-h incubation of dystrophic (mdx) ventricular cardiomyocytes with EMPA significantly increases peak INa in a concentration-dependent manner (EC50 = 94 nM). The enhancing effect on peak INa also occurred in dystrophic cardiac Purkinje fibers, as well as in dystrophic (DMDmdx) rat cardiomyocytes, and was also exerted by other SGLT2 inhibitors. Immunofluorescence studies suggested that chronic EMPA treatment fully restores wild-type Nav1.5 plasma membrane expression in mdx cardiomyocytes. Peak INa enhancement by EMPA depended on functional anterograde trafficking of Nav1.5. The local anesthetic mexiletine, a well-known pharmacological chaperone of Nav1.5, enhanced peak INa in a similar manner to EMPA. Furthermore, mutation of human Nav1.5 at a site important for local anesthetic binding (Y1767A) completely abolished the ability of both EMPA and mexiletine to enhance peak INa. Finally, the importance of Y1767 for drug-induced modulation of peak INa was confirmed by molecular docking simulations. Our findings suggest that EMPA acts as a pharmacological chaperone of Nav1.5 channels. Its chronic administration may reduce arrhythmia vulnerability in patients with DMD and other arrhythmogenic pathologies associated with diminished peak INa.NEW & NOTEWORTHY Dystrophin deficiency in cardiomyocytes leads to diminished peak Na currents. These can be fully rescued by long-term treatment with empagliflozin via pharmacochaperoning of Nav1.5 channels.
Keywords: Duchenne muscular dystrophy; arrhythmias; cardiomyocyte sodium currents; empagliflozin; pharmacochaperone.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Jimenez-Vazquez EN, Arad M, Macías Á, Vera-Pedrosa ML, Cruz FM, Gutierrez LK, Cuttitta AJ, Monteiro da Rocha TJ, Herron TJ, Ponce-Balbuena D, Guerrero-Serna G, et al. SNTA1 gene rescues ion channel function and is antiarrhythmic in cardiomyocytes derived from induced pluripotent stem cells from muscular dystrophy patients. eLife. 2022;11:e76576. doi: 10.7554/eLife.76576. - DOI - PMC - PubMed
-
- Perloff JK. Cardiac rhythm and conduction in Duchenne’s muscular dystrophy: a prospective study of 20 patients. J Am Coll Cardiol. 1984;3:1263–1268. - PubMed
-
- Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res. 2006;99:407–414. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

