Predicting ovarian function loss after chemotherapy and anti-HER2 therapy in young breast cancer patients
- PMID: 40796347
- PMCID: PMC12597502
- DOI: 10.1093/jnci/djaf198
Predicting ovarian function loss after chemotherapy and anti-HER2 therapy in young breast cancer patients
Abstract
Background: The ability to predict ovarian function loss after anticancer treatment is important for appropriate oncofertility counseling and to aid in therapy decision-making for young women with early breast cancer (eBC).
Methods: This biomarker analysis of the BETH (NCT00625898) and KAITLIN (NCT01966471) randomized trials investigated anti-Müllerian hormone (AMH) use, alone and combined with follicle stimulating hormone (FSH) and estradiol (E2), for predicting ovarian function loss following currently adopted chemotherapy and anti-HER2 therapy in premenopausal women with HER2-positive eBC. Serum samples were centrally tested measuring AMH, FSH, and E2 using Roche Elecsys assays.
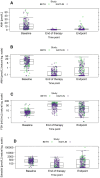
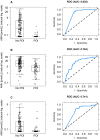
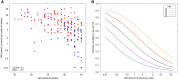
Results: Among 194 included patients (BETH: n = 62; KAITLIN: n = 132), AMH values declined from baseline median 8.44 pmol L-1 to undetectable levels (<0.07 pmol L-1) at the end of therapy, with partial recovery at 36 months (median 0.14 pmol L-1). AMH measured at baseline was predictive of ovarian loss (area under the ROC curve [AUC] = 0.784). Addition of age to AMH slightly improved AUC to 0.800. AMH measured at the end of therapy had AUC 0.741, which increased to 0.785 with addition of age. The combination of AMH at baseline and end of therapy increased prediction to 0.808 and with addition of age to 0.820. Addition of baseline FSH and E2 did not improve prediction in any analysis.
Conclusions: These results support the use of pretreatment measurement of AMH in predicting ovarian function loss in premenopausal women with HER2-positive eBC receiving chemotherapy and anti-HER2 therapy. Measurement of AMH at the end of treatment had reduced accuracy than pretreatment but in combination added slightly to the value of pretreatment sampling.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
M.L. reported an advisory role for Roche, Lilly, Novartis, AstraZeneca, Pfizer, Seagen, Gilead, MSD, Menarini, Nordic Pharma and Exact Sciences; speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo, Menarini, and Takeda; travel grants from Gilead, Roche and Daiichi Sankyo; research funding (to the Institution) from Gilead. M.L., who is a
Figures
Comment in
-
RecOVARY? Using anti-Müllerian hormone to predict ovarian function after anti-HER2 therapy for early breast cancer.J Natl Cancer Inst. 2025 Nov 1;117(11):2158-2160. doi: 10.1093/jnci/djaf247. J Natl Cancer Inst. 2025. PMID: 41005288 No abstract available.
References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. - PubMed
-
- Paluch-Shimon S, Cardoso F, Partridge AH, et al. ESO-ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann Oncol. 2022;33:1097-1118. - PubMed
-
- Lambertini M, Peccatori FA, Demeestere I, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31:1664-1678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

