Andrographolide promotes the ex vivo expansion of CD34+ hematopoietic stem cells derived from human umbilical cord blood
- PMID: 40796650
- PMCID: PMC12343817
- DOI: 10.1038/s41598-025-15647-9
Andrographolide promotes the ex vivo expansion of CD34+ hematopoietic stem cells derived from human umbilical cord blood
Abstract
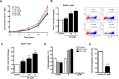
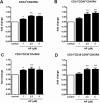
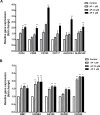
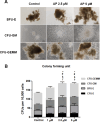
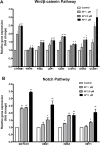
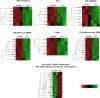
Umbilical cord blood (UCB) units are an alternative source of human hematopoietic stem cells (HSCs) for allogeneic stem cell transplants. A large quantity of HSCs is needed but the low number of accessible cells from UCB has been a significant limitation. Improving the ex vivo growth of HSCs while preserving their functioning is required. Here, we report that andrographolide (AP) enhanced the expansion of human UCB-derived HSCs (HSPCs) and pro-moted primitive HSCs (CD34+CD38-CD90+). AP also improved HSC functionality, evidenced by increased growth of colony-forming units and multilineage differentiation. AP upregulated genes involved in the Wnt/β-catenin and Notch signaling pathways. AP also modulated signaling pathways involved in HSC self-renewal, proliferation, survival, and differentiation, demonstrated by Nanostring analysis. The results of this study suggest that andrographolide enhances ex vivo UCB-HSC expansion while maintaining functionality and has potential for treatment of hematological diseases.
Keywords: Andrographolide; Ex vivo expansion; Hematologic disorders; Hematopoietic stem cell transplantation; Hematopoietic stem cells (HSCs); Umbilical cord blood (UCB).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Mesenchymal stem/stromal cells from human pluripotent stem cell-derived brain organoid enhance the ex vivo expansion and maintenance of hematopoietic stem/progenitor cells.Stem Cell Res Ther. 2024 Mar 5;15(1):68. doi: 10.1186/s13287-023-03624-w. Stem Cell Res Ther. 2024. PMID: 38443990 Free PMC article.
-
Umbilical cord blood derived cell expansion: a potential neuroprotective therapy.Stem Cell Res Ther. 2024 Jul 29;15(1):234. doi: 10.1186/s13287-024-03830-0. Stem Cell Res Ther. 2024. PMID: 39075614 Free PMC article. Review.
-
Endogenous Hydrogen Sulphide Promotes the Ex Vivo Expansion of Haematopoietic Stem Cells by Regulating the Activation of the JAK2/STAT3 Pathway.Immunology. 2025 Aug;175(4):534-543. doi: 10.1111/imm.13935. Epub 2025 May 22. Immunology. 2025. PMID: 40405487
-
The Evaluation of Mass/DNA Copy Number of Mitochondria in Umbilical Cord Blood-derived Hematopoietic Stem Cells Cocultured with MSCs.Indian J Hematol Blood Transfus. 2024 Oct;40(4):638-646. doi: 10.1007/s12288-024-01774-2. Epub 2024 Apr 15. Indian J Hematol Blood Transfus. 2024. PMID: 39469179
-
Clinical Outcomes of Umbilical Cord Blood Transplantation Using Ex Vivo Expansion: A Systematic Review and Meta-Analysis of Controlled Studies.Transplant Cell Ther. 2023 Feb;29(2):129.e1-129.e9. doi: 10.1016/j.jtct.2022.11.007. Epub 2022 Nov 15. Transplant Cell Ther. 2023. PMID: 36396108
References
-
- Alexander, T. & Greco, R. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the autoimmune diseases working party (ADWP) of the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant.57, 1055–1062. 10.1038/s41409-022-01702-w (2022). - PMC - PubMed
-
- Liao, Y., Geyer, M. B., Yang, A. J. & Cairo, M. S. Cord blood transplantation and stem cell regenerative potential. Exp. Hematol.39, 393–412. 10.1016/j.exphem.2011.01.002 (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

