The mitochondrial E3 ligase MAPL SUMOylates Drp1 to facilitate mitochondrial fission in intervertebral disc degeneration
- PMID: 40796734
- PMCID: PMC12343876
- DOI: 10.1038/s41413-025-00449-6
The mitochondrial E3 ligase MAPL SUMOylates Drp1 to facilitate mitochondrial fission in intervertebral disc degeneration
Abstract
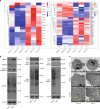
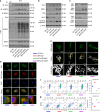
Intervertebral disc degeneration (IVDD) is the primary contributor to a range of spinal diseases. Dynamin-related protein 1 (Drp1)-mediated mitochondrial fission has recently been identified as a new cause of nucleus pulposus cell (NPC) death and IVDD, but the underlying mechanisms remain unclear. Although the effects of Drp1 phosphorylation in IVDD have been studied, it is currently unknown if small ubiquitin-like modifications (SUMOylation) of Drp1 regulate IVDD. This study aimed to investigate the functions and mechanisms of mitochondria-anchored protein ligase (MAPL), a mitochondrial SUMO E3 ligase, during IVDD progression. The expression of genes related to SUMOylation and mitochondrial dynamics in TNF-α-stimulated NPCs was analysed via RNA sequencing. The levels of total and mitochondrial SUMO1 conjugates were elevated with MAPL upregulation in TNF-α-treated NPCs. Additionally, mitochondrial fragmentation and dysfunction were induced by TNF-α stimulation. MAPL overexpression promoted mitochondrial SUMOylation and SUMO1 modification of Drp1, thereby facilitating the mitochondrial translocation of Drp1 and mitochondrial fission. MAPL-induced ROS accumulation and ΔΨm loss led to increased NPC apoptosis. Mutation of the SUMO-acceptor lysine residues of Drp1 hindered its SUMOylation and rescued the mitochondrial phenotypes caused by MAPL. SENP5 overexpression phenocopied MAPL silencing, negatively modulating the SUMO1 modification of Drp1 and mitochondrial fission in NPCs. In a rat IVDD model, forced expression of MAPL by using an adeno-associated virus (AAV) vector aggravated IVD tissue damage, whereas the knockdown of MAPL delayed IVDD progression. Our findings highlight the importance of SUMOylation in IVDD. The inhibition of MAPL-mediated Drp1 SUMOylation alleviates mitochondrial fission and limits IVDD development, providing a potential strategy for IVDD treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Qi, M. et al. Comparison of clinical outcomes between cervical disc arthroplasty and anterior cervical discectomy and fusion for the treatment of single-level cervical spondylosis: a 10-year follow-up study. Spine J.23, 361–368 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

