COUP-TFII-mediated reprogramming of the vascular endothelium counteracts tumor immune evasion
- PMID: 40796746
- PMCID: PMC12343902
- DOI: 10.1038/s41467-025-62399-1
COUP-TFII-mediated reprogramming of the vascular endothelium counteracts tumor immune evasion
Abstract
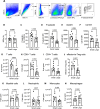
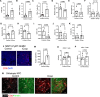
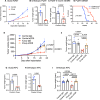
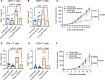
T cell scarcity in tumor tissues poses a critical challenge to cancer immunotherapy. Here we manipulate the tumor vasculature, an essential regulator of immune cell trafficking, to reinvigorate anti-tumor T cell responses in "cold" tumors. We show that ectopic pan-endothelial expression of COUP-TFII, a master transcription factor for venous development, induces molecular programs of post-capillary venules in tumor endothelium. Venular reprogramming selectively promotes T cell recruitment into tumors, inhibits tumor growth in mouse models of breast and pancreatic cancers, and sensitizes tumors to immune checkpoint blockade and adoptive T cell transfer therapies. Mechanistic studies show that enhanced recruitment of anti-tumor T cells and tumor inhibition are mediated by COUP-TFII-induced vascular adhesion receptors. Our study supports a pivotal role of vascular endothelial cells in governing tumor immune evasion, and proposes venular reprogramming as a therapeutic strategy to bolster anti-tumor immunity and immunotherapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Ochoa de Olza, M., Navarro Rodrigo, B., Zimmermann, S. & Coukos, G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol.21, e419–e430 (2020). - PubMed
-
- Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science348, 74–80 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA228019/CA/NCI NIH HHS/United States
- T32 HL098049/HL/NHLBI NIH HHS/United States
- S10 OD021763/OD/NIH HHS/United States
- AI130471/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- CA228019/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

