Conductivity hysteresis in MXene driven by structural dynamics of nanoconfined water
- PMID: 40796749
- PMCID: PMC12343936
- DOI: 10.1038/s41467-025-62892-7
Conductivity hysteresis in MXene driven by structural dynamics of nanoconfined water
Abstract
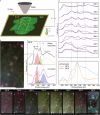
Water under 2D confinement exhibits unique structural and dynamic behaviors distinct from bulk water, including phase transitions and altered hydrogen-bonding networks, making it of great scientific interest. While confinement in 2D materials like graphene, mica, or hexagonal boron nitride has been reported, their lack of intrinsic hydrophilicity or metallic conductivity limits their suitability for probing the interplay between confined water and electronic transport. MXenes, a family of 2D transition metal carbides and nitrides, overcome these limitations by combining high metallic conductivity (~104 S cm-1) with hydrophilicity, offering a unique platform to investigate confined water dynamics and their influence on electronic properties. Here, we show that temperature and confinement drive structural transitions of water within MXene interlayers, including the formation of localized ice clusters, amorphous ice, and dynamic hydrogen-bonded networks. These transformations disrupt stacking order, inducing a reversible metal-to-semiconductor transition and conductivity hysteresis in MXene films. Upon heating to 340 K, the dissociation of ice clusters restores interlayer spacing and metallic behavior. Our findings experimentally establish MXenes as an exceptional platform for studying the phase change of confined water, offering new insights into how nanoscale water dynamics modulate electronic properties and enabling the design of advanced devices with tunable interlayer interactions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Kolesnikov, A. I. et al. Quantum tunneling of water in beryl: a new state of the water molecule. Phys. Rev. Lett.116, 167802 (2016). - PubMed
-
- Mallamace, F. et al. The influence of water on protein properties. J. Chem. Phys.141, 165104 (2014). - PubMed
-
- Monroe, J. et al. Water structure and properties at hydrophilic and hydrophobic surfaces. Annu. Rev. Chem. Biomol. Eng.11, 523–557 (2020). - PubMed
-
- Raviv, U., Laurat, P. & Klein, J. Fluidity of water confined to subnanometre films. Nature413, 51–54 (2001). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

