Impact of Nano-Selenium supplementation on the JAK/STAT signaling pathway in major depressive disorder: a Triple-Blind, randomized controlled trial
- PMID: 40796838
- PMCID: PMC12341080
- DOI: 10.1186/s12888-025-07213-4
Impact of Nano-Selenium supplementation on the JAK/STAT signaling pathway in major depressive disorder: a Triple-Blind, randomized controlled trial
Abstract
Background: Major depressive disorder (MDD) is a prevalent mental health condition, wherein the JAK/STAT signaling pathway serves as a potent cellular mechanism implicated in its pathophysiology. Increased expression of JAK2, STAT3, and subsequently IDO1 genes appears to be linked to depressive symptoms. With their antioxidant capabilities and improved absorption due to the nano formula, selenium nanoparticles could potentially modulate this molecular pathway. This study aimed to assess the impact of nano-selenium supplementation on the expression of JAK2, STAT3, and IDO1 genes in patients with MDD.
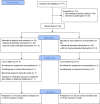
Methods: A triple-blind, randomized, placebo-controlled trial was conducted at the Psychosomatic Clinic of Imam Khomeini Hospital Complex. A total of 50 participants, newly diagnosed with MDD were randomized to either a nano-selenium (55 µg/day) or placebo group for 12 weeks. All participants were receiving their standard treatment (sertraline 50 mg/day). Blood samples were collected at baseline and post-intervention to measure the gene expression using RT-qPCR.
Results: At the end of the study, both groups showed reductions in JAK2 and STAT3 relative gene expression after 12 weeks (P < 0.05). Although the reduction was more in the nano-selenium group, the between-group differences were not statistically significant.
Conclusions: This study is the first to examine nano-selenium as a novel potential adjunct treatment for MDD. Though the degree of reduction in JAK2 and STAT3 levels was greater within the nano-selenium group, it appears that additional investigations are needed to elucidate its effects.
Trial registration: The research received approval from the Research Ethics Committees of Iran University of Medical Sciences (Approval ID: IR IUMS.REC.1402.206, dated 2023-06-13) and was duly registered with the Iranian Registry of Clinical Trials (IRCT; registration number: IRCT20091114002709N62, dated 2023-07-29).
Keywords: Depressive disorder; Randomized controlled trial; Selenium; Trace elements.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Research Ethics Committee of Iran University of Medical Sciences (Approval ID: IR.IUMS.REC.1402.206, dated 2023-06-13) and registered with the Iranian Registry of Clinical Trials (IRCT registration number: IRCT20091114002709N62, dated 2023-07-29). Written informed consent was obtained from all participants prior to their enrollment in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Marx W, Penninx BWJH, Solmi M, Furukawa TA, Firth J, Carvalho AF, Berk M. Major depressive disorder. Nat Reviews Disease Primers. 2023;9(1):44. - PubMed
-
- Shariq AS, Brietzke E, Rosenblat JD, Pan Z, Rong C, Ragguett R-M, Park C, McIntyre RS. Therapeutic potential of JAK/STAT pathway modulation in mood disorders. Rev Neurosci. 2018;30(1):1–7. - PubMed
-
- Gulbins A, Grassmé H, Hoehn R, Kohnen M, Edwards MJ, Kornhuber J, Gulbins E. Role of Janus-Kinases in major depressive disorder. Neurosignals. 2016;24(1):71–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

