Surgery-specific patterns of perioperative amino acid administration and associated acute kidney injury risk: a large-scale retrospective cohort study
- PMID: 40796875
- PMCID: PMC12341336
- DOI: 10.1186/s13741-025-00573-1
Surgery-specific patterns of perioperative amino acid administration and associated acute kidney injury risk: a large-scale retrospective cohort study
Abstract
Background: Recent trials demonstrated renoprotective effects of amino acid infusion in cardiac surgery patients, but real-world utilization patterns and outcomes across surgical specialties remain unknown. We investigated perioperative amino acid administration patterns and associated acute kidney injury (AKI) risk across different surgical populations.
Methods: Retrospective cohort study using the INSPIRE database (2011-2020) from Seoul National University Hospital. Adult patients undergoing surgery with ≥ 24-h stays were included. Amino acid preparations were identified by ATC codes, and AKI was defined by KDIGO criteria. Primary outcomes were AKI incidence and utilization patterns across surgical departments.
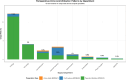
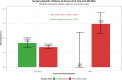
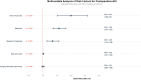
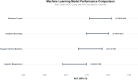
Results: Among 22,972 patients, 899 (3.9%) received peri-operative amino acid preparations with an overall AKI incidence of 3.7%. Utilization varied 60-fold across departments (0.2-11.5%). Surgery-specific patterns emerged: cardiac surgery showed no AKI events in amino acid users (0/50) versus 4.2% in non-users (p = 0.267), while non-cardiac surgery demonstrated increased AKI risk with amino acid use (7.4% vs 3.4%; RR = 2.16, 95% CI 1.65-2.85, p < 0.001). Multivariable analysis confirmed amino acid use as an independent AKI predictor (OR = 2.01, 95% CI 1.52-2.60). Machine learning analysis confirmed amino acids as the strongest AKI predictor, with Random Forest achieving superior performance (AUC-ROC 0.782) and revealing significant non-linear interactions. Propensity score matching (799 pairs) confirmed the association (OR = 1.63, 95% CI 1.05-2.52, p = 0.029).
Conclusions: Perioperative amino acid administration demonstrates surgery-specific patterns with differential AKI associations. These findings suggest that surgery-specific factors should be considered when developing amino-acid protocols, although causality cannot be established from this observational study.
Keywords: Acute kidney injury; Amino acids; Perioperative care; Practice variation; Surgery-specific.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Seoul National University Hospital Institutional Review Board (H-2210-078-1368). Informed consent was waived due to the retrospective nature and use of de-identified data. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass.Cochrane Database Syst Rev. 2015 Mar 11;2015(3):CD010480. doi: 10.1002/14651858.CD010480.pub2. Cochrane Database Syst Rev. 2015. PMID: 25758322 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Risk of acute kidney injury after lower urinary tract reconstruction with early NSAID therapy: A propensity matched retrospective analysis.J Pediatr Urol. 2024 Oct;20(5):911-920. doi: 10.1016/j.jpurol.2024.07.005. Epub 2024 Jul 16. J Pediatr Urol. 2024. PMID: 39089953
References
-
- Baiardo Redaelli M, Monaco F, Bradic N, et al. Amino acid infusion for kidney protection in cardiac surgery patients with chronic kidney disease: a secondary analysis of the PROTECTION trial. Anesthesiology. 2025;142(5):818–28. - PubMed
-
- Jiang W, Shi K, Shao J, et al. Protective effect of intravenous amino acid on kidney function: A systematic review and meta-analysis of randomized controlled trials. J Crit Care. 2025;85:154937. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

