3D cell-laden scaffold printed with brain acellular matrix bioink
- PMID: 40796885
- PMCID: PMC12344831
- DOI: 10.1186/s12951-025-03644-z
3D cell-laden scaffold printed with brain acellular matrix bioink
Abstract
Background: Intracerebral hemorrhage (ICH) is a severe neurological disorder characterized by bleeding within the brain tissue, typically associated with factors such as hypertension, cerebrovascular disease, and trauma. The transplantation of human umbilical cord-derived mesenchymal stem cells (hUCMSCs) has demonstrated promising effects in restoring neurological function in ICH rats; however, limited retention of these cells significantly impedes their efficacy. To address this limitation, we developed a bioink composed of decellularized extracellular matrix (dECM) and hUCMSCs, which was synthesized into 3D cell-laden scaffold through 3D bioprinting. This approach aims to extend the retention of hUCMSCs and create an early vascular microenvironment, thereby partially compensating for the drawbacks of hUCMSC transplantation and improving neurological function in ICH rats.
Methods: This study aimed to explore the use of a bioink formed by mixing 15% gelatin and 3% sodium alginate with a dECM solution, in conjunction with hUCMSCs, for 3D bioprinting of 3D cell-laden scaffold. The viscosity, morphology, and biocompatibility of the bioink were characterized using rheological analysis, scanning electron microscopy (SEM), and hematoxylin and eosin (HE) staining. Following printing, a live/dead assay kit was employed to assess the viability of hUCMSCs within the 3D cell-laden scaffold. ICH model rats were randomly assigned to four groups: (1) SHAM group; (2) ICH group; (3) ICH + 3D biological scaffold group; and (4) ICH + 3D cell-laden scaffold group.
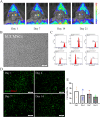
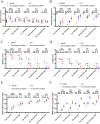
Results: hUCMSCs exhibited a higher retention rate within the 3D bioprinted 3D cell-laden scaffold. HE staining, immunohistochemistry, and immunofluorescence results indicated that the 3D biological scaffold encapsulating hUCMSCs had a significant impact on the vascularization of the printed 3D cell-laden scaffold. Furthermore, 3D cell-laden scaffold improved nerve function and promoted angiogenesis in rats with cerebral hemorrhage better than 3D biological scaffolds.
Conclusion: Our results suggest that 3D bioprinted 3D cell-laden scaffold hold great potential for restoring impaired neurological function in ICH rats.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12951-025-03644-z.
Keywords: 3D bioprinting; 3D cell-laden scaffold; Angiogenesis; Decellularized extracellular matrix; Human umbilical cord-derived mesenchymal stem cells.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Ethics Committee of the Second Hospital of Hebei Medical University (Approval Number: 2024-R240 and 2024-R251). Consent for publication: All authors have read the manuscript and provided their consent for the submission. Competing interests: The authors declare no competing interests.
Figures





References
-
- Li L, Xu Y, Peng Q, Huang P, Duan X, Wang M, Jiang Y, Wang J, Periasamy S, Hsieh DJ, Chang KC. Biocompatible acellular dermal Matrix-Based neuromorphic device with ultralow voltage, ion channel emulation, and synaptic forgetting visualization computation. ACS Nano. 2024;18(45):31309–22. 10.1021/acsnano.4c10383. Epub 2024 Oct 31. - DOI - PubMed
-
- Latchoumane CV, Betancur MI, Simchick GA, Sun MK, Forghani R, Lenear CE, Ahmed A, Mohankumar R, Balaji N, Mason HD, Archer-Hartmann SA, Azadi P, Holmes PV, Zhao Q, Bellamkonda RV, Karumbaiah L. Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci Adv. 2021;7(10):eabe0207. 10.1126/sciadv.abe0207. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

