Population genomics reveal multiple independent origins of pesticide resistance in the polyphagous pest, Tetranychus urticae
- PMID: 40797102
- PMCID: PMC12344295
- DOI: 10.1038/s42003-025-08658-9
Population genomics reveal multiple independent origins of pesticide resistance in the polyphagous pest, Tetranychus urticae
Abstract
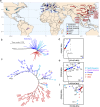
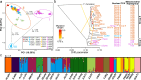
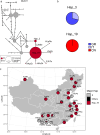
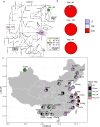
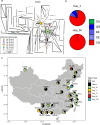
The rapid evolution of pesticide resistance imposes great pressure on food production. However, how resistance alleles arise and spread across field populations remains largely understood. Here, we study the evolutionary trajectories of resistance alleles in Tetranychus urticae, a rapidly evolving pest. We sequence the genomes of 258 T. urticae females collected from China. Combined with global reference genomic data, we examine the evolutionary origin(s) of 18 mutations across 10 target-site genes and analyze the global population genetic structure using genome-wide SNPs. Our findings reveal a striking prevalence of multiple independent origins of resistance mutations, with only two of 18 mutations showing an apparent single origin. Population structure and haplotype analyses point to an important role of gene flow in the spread of resistance alleles. Selection analyses reveal pesticide-driven sweeps affecting genetic diversity. These findings advance our understanding of the rapid adaptation of arthropod herbivores to extreme selective pressure.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Recurrent mutations drive the rapid evolution of pesticide resistance in the two-spotted spider mite Tetranychus urticae.Elife. 2025 Aug 11;14:RP106288. doi: 10.7554/eLife.106288. Elife. 2025. PMID: 40788297 Free PMC article.
-
Malaria parasite population genomics during an elimination program in Eastern Myanmar.bioRxiv [Preprint]. 2025 May 26:2025.05.21.655408. doi: 10.1101/2025.05.21.655408. bioRxiv. 2025. PMID: 40661555 Free PMC article. Preprint.
-
Independent Genetic Mapping Experiments Identify Diverse Molecular Determinants of Host Adaptation in a Generalist Herbivore.Mol Ecol. 2025 Aug;34(16):e17618. doi: 10.1111/mec.17618. Epub 2024 Dec 16. Mol Ecol. 2025. PMID: 39676612
-
Genomic investigations of successful invasions: the picture emerging from recent studies.Biol Rev Camb Philos Soc. 2025 Jun;100(3):1396-1418. doi: 10.1111/brv.70005. Epub 2025 Feb 16. Biol Rev Camb Philos Soc. 2025. PMID: 39956989 Free PMC article.
-
Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin.Cochrane Database Syst Rev. 2022 May 18;5(5):CD014841. doi: 10.1002/14651858.CD014841.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583175 Free PMC article.
References
-
- Baucom, R. S., Iriart, V., Kreiner, J. M. & Yakimowski, S. Resistance evolution, from genetic mechanism to ecological context. Mol. Ecol.30, 5299–5302 (2021). - PubMed
-
- De Rouck, S., Inak, E., Dermauw, W. & Van Leeuwen, T. A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem. Mol. Biol.159, 103981 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

