Gut microbiota and urine metabolomics signature in autism spectrum disorder children from Southern China
- PMID: 40797237
- PMCID: PMC12341299
- DOI: 10.1186/s12887-025-05922-z
Gut microbiota and urine metabolomics signature in autism spectrum disorder children from Southern China
Abstract
Background and aim: Autism spectrum disorder (ASD) is a neurodevelopmental disorder that may have long-term effects on individual development, family functioning, and social integration. This study aimed to determine the gut microbiota and urine metabolomics signature and identify the regional characteristics in ASD from Southern China.
Methods: We conducted a cohort study of 88 well-characterized participants from Guangxi Zhuang Autonomous Region in Southern China. Gut microbiota and urine metabolomics signature was explored by 16 S rRNA sequences and untargeted metabolomic profiles respectively.
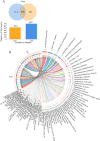
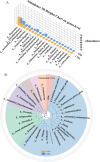
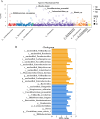
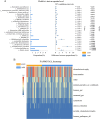
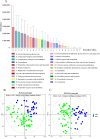
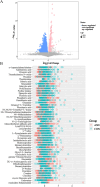
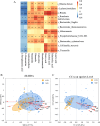
Results: The gut microbial α-diversity of ASD were significantly lower than healthy controls. The β-diversity analysis indicated that the community structure in ASD group was obviously distinctive. Significant microbiota enriched in 5 sensitive species, Faecalibacterium prausnitzii, Bifidobacterium catenulatum, Blautia obeum, Lachnoclostridium sp., and Blautia sp. in ASD children. In addition, functional analysis of the gut microbiota revealed that the ATP-binding cassette and ABC-2 type transport system ATP-binding protein were closely associated with ASD. Notably, microbiota showing a positive correlation with Androstenedione, Stearamide, Oleamide, Cadaverine, Hexadecanamide, Orotic acid, Linoleic acid, Palmitoleic acid, Lauric acid, suggesting a potential association with the Arginine and proline metabolism pathway.
Conclusion: This study found lower α-diversity, unique β-diversity, enriched species, and positive correlations between microbiota and Arginine/Proline metabolis, which demonstrated typical signature of microbiota and metabolites discriminated Zhuang ethnic group ASD children of regional characteristics.
Keywords: Autism spectrum disorder; Guangxi Zhuang autonomous region; Gut microbiota; Urine metabolomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (NO.2021-KT-003). All parents of participants in this study have provided written informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, Furnier SM, Hallas L, Hall-Lande J, Hudson A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years-Autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70:1–16. - PMC - PubMed
-
- Maenner MJ, Warren Z, Williams AR, Amoakohene E, Bakian AV, Bilder DA, Durkin MS, Fitzgerald RT, Furnier SM, Hughes MM, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR Surveill Summ. 2023;72:1–14. - PMC - PubMed
-
- Kang-Yi CD, Grinker RR, Mandell DS. Korean culture and autism spectrum disorders. J Autism Dev Disord. 2013;43:503–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

