Gut microbiota links to cognitive impairment in bipolar disorder via modulating synaptic plasticity
- PMID: 40797316
- PMCID: PMC12345090
- DOI: 10.1186/s12916-025-04313-6
Gut microbiota links to cognitive impairment in bipolar disorder via modulating synaptic plasticity
Abstract
Background: Cognitive impairment is an intractable clinical manifestation of bipolar disorder (BD), but its underlying mechanisms remain largely unexplored. Preliminary evidence suggests that gut microbiota can potentially influence cognitive function by modulating synaptic plasticity. Herein, we characterized the gut microbial structure in BD patients with and without cognitive impairment and explored its influence on neuroplasticity in mice.
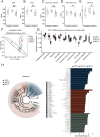
Methods: The gut structure of microbiota in BD without cognitive impairment (BD-nCI) patients, BD with cognitive impairment (BD-CI) patients, and healthy controls (HCs) were characterized, and the correlation between specific bacterial genera and clinical parameters was determined. ABX-treated C57 BL/J male mice were transplanted with fecal microbiota from BD-nCI, BD-CI patients or HCs and subjected to behavioral testing. The change of gut microbiota in recipient mice and its influence on the dendritic complexity and synaptic plasticity of prefrontal neurons were examined. Finally, microbiota supplementation from healthy individuals in the BD-CI mice was performed to further determine the role of gut microbiota.
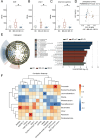
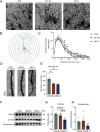
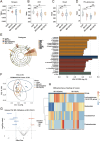
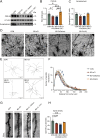
Results: 16S-ribosomal RNA gene sequencing reveals that gut microbial diversity and composition are significantly different among BD-nCI patients, BD-CI patients, and HCs. The Spearman correlation analysis suggested that glucose metabolism-related bacteria, such as Prevotella, Faecalibacterium, and Roseburia, were correlated with cognitive impairment test scores, and inflammation-related bacteria, such as Lachnoclostridium and Bacteroides, were correlated with depressive severity. Fecal microbiota transplantation resulted in depression-like behavior, impaired working memory and object recognition memory in BD-CI recipient mice. Compared with BD-nCI mice, BD-CI mice exhibited more severely impaired object recognition memory, along with greater reductions in dendritic complexity and synaptic plasticity. Supplementation of gut microbiota from healthy individuals partially reversed emotional and cognitive phenotypes and neuronal plasticity in BD-CI mice.
Conclusions: This study first characterized the gut microbiota in BD-CI patients and highlighted the potential role of gut microbiota in BD-related cognitive deficits by modulating neuronal plasticity in mice model.
Keywords: Bipolar disorder; Cognitive impairment; Gut microbiota; Neuroplasticity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Review Board of the First Affiliated Hospital, School of Medicine of Zhejiang University (reference number #2017–397). All participants provided written informed consent. All animal experiments were approved by the Animal Experimental Ethical Inspection of the First Affiliated Hospital, Zhejiang University School of Medicine (Reference number 2024–376). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Grants and funding
- 82201676/National Natural Science Foundation of China
- 82471542/National Natural Science Foundation of China
- No. JNL-2023001B/Research Project of Jinan Microecological Biomedicine Shandong Laboratory
- 2023YFC2506200/National Key Research and Development Program of China
- 2021C03107/Zhejiang Provincial Key Research and Development Program
- 2023ZFJH01-01/Fundamental Research Funds for the Central Universities
- 2024ZFJH01-01/Fundamental Research Funds for the Central Universities
- No. 2021R52016/Leading Talent of Scientific and Technological Innovation - "Ten Thousand Talents Program" of Zhejiang Province
- 2022KTZ004/Chinese Medical Education Association
LinkOut - more resources
Full Text Sources
Medical

