Role of vitamin D as adjuvant therapy on multiple sclerosis: an updated systematic review and meta-analysis of randomized controlled trials
- PMID: 40797337
- PMCID: PMC12341198
- DOI: 10.1186/s40001-025-02981-x
Role of vitamin D as adjuvant therapy on multiple sclerosis: an updated systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Multiple sclerosis (MS) is the most common demyelinating disorder affecting the central nervous system, with multiple risk factors suggested to be involved in the pathogenesis. Many studies have linked vitamin D deficiency to an increased risk of MS. This review aims to comprehensively evaluate the published randomized clinical trials (RCTs) of vitamin D supplements as add-on therapy for MS patients.
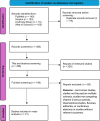
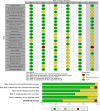
Methods: We systematically searched the Web of Science, Scopus, PubMed, and Cochrane databases up to August 2024 for the published RCTs evaluating the use of vitamin D for MS in adults. All included studies were screened and abstracted independently by two authors. Radiological and clinical outcomes were extracted, and the meta-analysis was conducted using Review Manager 5.4.
Results: Our meta-analysis, which included 21 studies with 1,903 patients (20.1% males), found that vitamin D supplementation significantly reduced expanded disability status scale scores (MD = - 0.17, p = 0.03), relapse rates (OR = 0.66, p = 0.02), and new T2 lesion formation (MD = - 0.48, p = 0.03) in patients with MS compared to controls, with minimal to no heterogeneity. However, there was no effect on the annual relapse rate (p = 0.81), timed 25-foot walk (p = 0.54), fatigue severity, or quality of life. Subgroup analysis indicated a relapse rate reduction only in those treated for more than 12 months (OR = 0.53, p = 0.003), suggesting duration-dependent benefits of vitamin D.
Conclusions: Vitamin D supplementation produces statistically significant-yet clinically modest-reductions in disability progression, relapses, and new T2-lesion formation without demonstrable effects on fatigue or quality of life. Accordingly, it should be considered a potentially helpful adjunct pending more definitive evidence. Larger, dose-stratified trials powered for clinically meaningful endpoints are still needed before vitamin D can be endorsed as an efficacy-proven disease-modifying therapy.
Keywords: Cholecalciferol; Disease-modifying therapy; Multiple sclerosis; Vitamin D.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Informed consent: Not applicable. Institutional review board statement: Not applicable. Competing interests: The authors declare no competing interests.
Figures




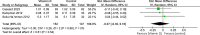

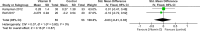
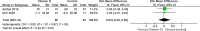
References
-
- Number of people with MS. Atlas of MS. https://atlasofms.org/map/global/epidemiology/number-of-people-with-ms. Accessed on 03 June 2025.
-
- Tafti D, Ehsan M, Xixis KL. Multiple sclerosis. In: Tafti D, editor. StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

