Population pharmacokinetics of bictegravir in real-world people with HIV
- PMID: 40798866
- PMCID: PMC12494133
- DOI: 10.1093/jac/dkaf297
Population pharmacokinetics of bictegravir in real-world people with HIV
Abstract
Objectives: Bictegravir is an integrase strand transfer inhibitor widely prescribed due to its good efficacy and safety profile. Its pharmacokinetic (PK) profile has not been described in real-world settings yet. This study aimed to characterize bictegravir population PK in people with HIV (PWH) and to identify covariates affecting its disposition.
Methods: Bictegravir concentrations were obtained from PWH as part of a therapeutic drug monitoring (TDM) follow-up performed at the Lausanne University Hospital, Switzerland, between July 2019 and July 2024. Demographic data, clinical data and co-medications were recorded during the routine Swiss HIV Cohort Study (SHCS) visits. A population PK model was developed using a non-linear mixed-effect approach with Monolix®.
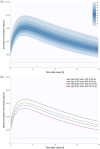
Results: A total of 708 steady-state plasma concentrations from 572 PWH were available for the analysis. A one-compartment model with first-order absorption and elimination best characterized bictegravir PK. Age and body weight were found to significantly affect bictegravir clearance, individuals of 51 years weighing 100 kg showing a 13% increase, and those aged 80 years weighing 70 kg a 11% decrease, relative to an individual weighing 70 kg and aged 51 years. These effects are not deemed clinically significant and do not warrant a clinical intervention.
Conclusions: Considering the good safety and efficacy profile of bictegravir, routine TDM is of limited value for bictegravir. However, TDM of bictegravir could be beneficial in case of suspected viral resistance or non-adherence, in special subpopulations (i.e. obese individuals and pregnant women), or to monitor drug-drug interactions.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- European Medicines Agency . Biktarvy epar public assessment report 2018. https://www.ema.europa.eu/en/documents/assessment-report/biktarvy-epar-p....
-
- Gilead Sciences . Biktarvy. U.S. Food and Drug administration. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210251Orig1s000M....
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ad....
-
- European AIDS Clinical Society . European AIDS Clinical Society Guidelines Version 10.0. https://www.eacsociety.org/media/2019_guidelines-10.0_final.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

