Control of Overly Secreted Tryptophanyl tRNA Synthetase Attenuates Sepsis Severity in a Porcine Model
- PMID: 40798912
- PMCID: PMC12408201
- DOI: 10.4062/biomolther.2025.071
Control of Overly Secreted Tryptophanyl tRNA Synthetase Attenuates Sepsis Severity in a Porcine Model
Abstract
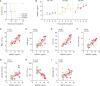
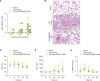
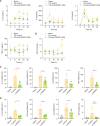
Sepsis is a leading cause of mortality in hospitals with a lack of reliable biomarkers and specialized therapeutics. Recently, highly secreted tryptophanyl-tRNA synthetase 1 (WARS1), an endogenous ligand for Toll-like receptor (TLR) 2 and TLR4, was found to be a potential theranostic target for hypercytokinemic severe sepsis. In this study, using the minipig sepsis model inoculated with cecum slurry, we demonstrated that increases in WARS1 levels were associated with severity of sepsis and showed strong correlations with RBC count and the levels of HGB, HCT, EPO, lactate, and PLT count in the acute phase of sepsis. Further, administration of the WARS1 neutralizing antibody to the septic minipigs inhibited the increase in the overall SOFA score with a significantly lower P/F ratio, which was accompanied by the suppression of proinflammatory cytokine and chemokine expressions as well as EPO production, a decrease in AST and ALT levels, and inflammatory immune cell infiltration in the lung. Taken together, these findings provide a novel insight into the pathophysiology of acute phase of sepsis and suggest the clinical application of WARS1 neutralizing therapeutics in the treatment of sepsis.
Keywords: Anti-WARS1 neutralizing antibody; Organ dysfunction; Sepsis; Severity biomarker; Tryptophanyl-tRNA synthetase (WARS1).
Conflict of interest statement
Mirim Jin is the founder and a shareholder of MirimGENE Co., Ltd., Incheon, South Korea. All authors declare no competing interests.
Figures



References
-
- Ahn Y. H., Park S., Choi J. J., Park B. K., Rhee K. H., Kang E., Ahn S., Lee C. H., Lee J. S., Inn K. S., Cho M. L., Park S. H., Park K., Park H. J., Lee J. H., Park J. W., Kwon N. H., Shim H., Han B. W., Kim P., Lee J. Y., Jeon Y., Huh J. W., Jin M., Kim S. Corrigendum: secreted tryptophanyl-tRNA synthetase as a primary defence system against infection. Nat. Microbiol. 2017;2:17015. doi: 10.1038/nmicrobiol.2017.15. - DOI - PubMed
-
- Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C. M., French C., Machado F. R., McIntyre L., Ostermann M., Prescott H. C., Schorr C., Simpson S., Wiersinga W. J., Alshamsi F., Angus D. C., Arabi Y., Azevedo L., Beale R., Beilman G., Belley-Cote E., Burry L., Cecconi M., Centofanti J., Coz Yataco A., De Waele J., Dellinger R. P., Doi K., Du B., Estenssoro E., Ferrer R., Gomersall C., Hodgson C., Hylander Moller M., Iwashyna T., Jacob S., Kleinpell R., Klompas M., Koh Y., Kumar A., Kwizera A., Lobo S., Masur H., McGloughlin S., Mehta S., Mehta Y., Mer M., Nunnally M., Oczkowski S., Osborn T., Papathanassoglou E., Perner A., Puskarich M., Roberts J., Schweickert W., Seckel M., Sevransky J., Sprung C. L., Welte T., Zimmerman J., Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. - DOI - PubMed
-
- Flisikowska T., Egli J., Flisikowski K., Stumbaum M., Kung E., Ebeling M., Schmucki R., Georges G., Singer T., Kurome M., Kessler B., Zakhartchenko V., Wolf E., Weber F., Schnieke A., Iglesias A. A humanized minipig model for the toxicological testing of therapeutic recombinant antibodies. Nat. Biomed. Eng. 2022;6:1248–1256. doi: 10.1038/s41551-022-00921-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

