Systematic Targeting of GD2-Positive Neuroblastoma Tumors With a Photooncolytic Phage Nanovector Platform
- PMID: 40799139
- PMCID: PMC12520574
- DOI: 10.1002/advs.202415356
Systematic Targeting of GD2-Positive Neuroblastoma Tumors With a Photooncolytic Phage Nanovector Platform
Abstract
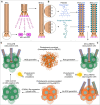
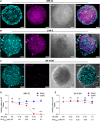
Disialoganglioside-GD2 is a key molecular target for Neuroblastoma (NB) immunotherapy based on the employment of GD2-targeting antibodies. However, about 50% of treated patients can experience tumor relapse due to limited immune-mediated cytotoxicity and poor antibody penetration into tumors. To address this problem, a tumor-penetrating photo-oncolytic phage nanovector platform is genetically and chemically developed that selectively targets GD2-expressing NB cells. The phage bioconjugates, functionalized with different photosensitizers, result in specific and selective oncolysis of GD2-positive NB cells upon light irradiation, without affecting GD2-negative ones. The photo-oncolytic phage vectors are shown to deeply penetrate into GD2-positive tumor spheroids in vitro, and to cross biological barriers in a zebrafish xenograft model, maintaining their ablation specificity upon irradiation. Finally, to overcome resistance from GD2 loss, often linked to poor prognosis, a CRISPRa strategy is introduced to reactivate GD2 expression in GD2-negative cells. The approach offers a minimally invasive and highly effective strategy, addressing unmet needs in NB therapy.
Keywords: CRISPRa; M13 bacteriophage biotherapeutics; neuroblastoma GD2; photodynamic therapy; zebrafish xenograft.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Matthay K. K., Maris J. M., Schleiermacher G., Nakagawara A., Mackall C. L., Diller L., Weiss W. A., Nat. Rev.: Dis. Prim. 2016, 2. - PubMed
- b) Steliarova‐Foucher E., Colombet M., Ries L. A., Moreno F., Dolya A., Bray F., Hesseling P., Shin H. Y., Stiller C. A., Lancet Oncol. 2017, 18, 719.
-
- a) Shendy N. A., Zimmerman M. W., Abraham B. J., Durbin A. D., Cell Rep. Med. 2022, 3, 100632; - PMC - PubMed
- b) Rodriguez‐Fos E., Planas‐Fèlix M., Burkert M., Puiggròs M., Toedling J., Thiessen N., Blanc E., Szymansky A., Hertwig F., Ishaque N., Beule D., Torrents D., Eggert A., Koche R. P., Schwarz R. F., Haase K., Schulte J. H., Henssen A. G., Cell Genom. 2023, 3, 100402. - PMC - PubMed
-
- Amoroso L., Haupt R., Garaventa A., Ponzoni M., Expert Opin. Invest. Drugs 2017, 26, 1281. - PubMed
-
- a) Jackson C., Modak S., LaQuaglia M., Kushner B., Gerstle J., Basu E., Cheung N., Iglesias‐Cardenas F., Wolden S., Int. J. Radiat. Oncol. Biol., Phys. 2024, 120, S49;
- b) Park J. R., Kreissman S. G., London W. B., Naranjo A., Cohn S. L., Hogarty M. D., Tenney S. C., Haas‐Kogan D., Shaw P. J., Kraveka J. M., JAMA, J. Am. Med. Assoc. 2019, 322, 746. - PMC - PubMed
-
- Ryan A. L., Akinkuotu A., Pierro A., Morgenstern D. A., Irwin M. S., J. Pediatr. Hematol./Oncol. 2020, 42, 1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
