TBX5 and CHD4 Coordinately Activate Atrial Cardiomyocyte Genes to Maintain Cardiac Rhythm Homeostasis
- PMID: 40799140
- PMCID: PMC12479101
- DOI: 10.1161/CIRCULATIONAHA.125.073833
TBX5 and CHD4 Coordinately Activate Atrial Cardiomyocyte Genes to Maintain Cardiac Rhythm Homeostasis
Abstract
Background: Atrial fibrillation, the most common sustained arrhythmia, affects 59 million individuals worldwide. The transcription factor TBX5 (T-box 5) is essential for normal atrial rhythm. Its inactivation causes loss of atrial cardiomyocyte (aCM) enhancer accessibility, looping, transcriptional identity, and spontaneous atrial fibrillation. TBX5 interacts with CHD4 (chromodomain helicase DNA-binding protein 4), a chromatin remodeling ATPase canonically associated with the NuRD (nucleosome remodeling and deacetylase) repressor complex.
Methods: We investigated mechanisms by which TBX5 regulates chromatin organization by studying mice with aCM-selective inactivation of TBX5 or CHD4. We integrated multiple genomics approaches including concurrent single-nucleus transcriptome and open chromatin profiling and genome-wide TBX5 and CHD4 chromatin occupancy assays.
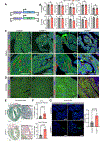
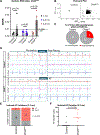
Results: We found that TBX5 recruits CHD4 to 33 170 genomic regions (TBX5-enhanced CHD4 sites). In addition to the canonical repressive activity of CHD4, we uncovered a CHD4 activator function predominantly at sites to which it was recruited by TBX5. TBX5-enhanced CHD4 recruitment increased local chromatin accessibility and promoted the expression of aCM identity genes. This mechanism of CHD4 recruitment by TBX5 was crucial for sinus rhythm; mice with CHD4 inactivation in aCMs had increased atrial fibrillation vulnerability. Assaying TBX5 binding in Chd4AKO atria demonstrated that CHD4 also promotes TBX5 binding at >10 000 genomic loci, including 3051 TBX5-enhanced CHD4 sites. Consistent with its requirement to maintain normal atrial rhythm, CHD4 was implicated in the regulation of 42 genes linked to atrial fibrillation in humans. Nine had the hallmarks of TBX5-dependent, CHD4-mediated transcriptional activation.
Conclusions: Our findings reveal that normal atrial rhythm requires CHD4, which activates and represses atrial genes in a context-dependent manner to maintain aCM gene expression, aCM identity, and atrial rhythm homeostasis.
Keywords: arrhythmias, cardiac; atrial fibrillation; transcription factors.
Conflict of interest statement
None.
Figures






References
-
- Wang Y, Wang J, Shi L, Chen X, Li D, Cui C, Yang K, Lu M, Huang J, Zhang L, et al. CIB2 is a novel endogenous repressor of atrial remodeling. Circulation. 2023;147:1758–1776. - PubMed
-
- Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, et al. 2023 ACC/AHA/ACCP/HRS guideline for the Diagnosis and Management of Atrial Fibrillation: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2024;149:e1–e156. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

