4'-Methoxyresveratrol Improves Hepatic Insulin Resistance Induced by a High-Fat-Diet via Anti-Oxidative-Stress Activity
- PMID: 40799306
- PMCID: PMC12341807
- DOI: 10.2147/DMSO.S535103
4'-Methoxyresveratrol Improves Hepatic Insulin Resistance Induced by a High-Fat-Diet via Anti-Oxidative-Stress Activity
Abstract
Background: Obesity and a high-fat diet (HFD) are key factors contributing to hepatic fat accumulation and type 2 diabetes mellitus (T2DM). Research indicates that oxidative stress induced by HFD mediates hepatic insulin resistance (HIR) through mechanisms such as promoting inflammation and lipid accumulation. Given that 4'-methoxyresveratrol (4-MR) exhibits remarkable anti-inflammatory and antioxidant activities, it is of great research significance to explore whether it can effectively counteract HFD-induced HIR by enhancing antioxidant stress.
Purpose: This study aimed to explore the effects of 4MR on liver oxidative damage and insulin sensitivity.
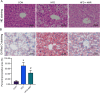
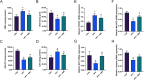
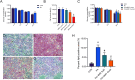
Methods: Mice were fed a HFD for 8 weeks, followed by an intraperitoneal glucose tolerance test to assess insulin resistance. The in-vivo experiments were divided into three groups, including the control group, the HFD group, and the HFD + 4MR group. After 6 weeks of 4MR treatment, blood and liver samples were analyzed for biochemical markers, lipid peroxidation, antioxidant capacity, and liver protein levels. Insulin-resistant HepG2 cells were treated with 4MR and evaluated for proliferation and toxicity.
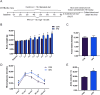
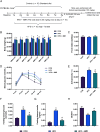
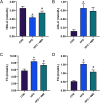
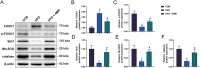
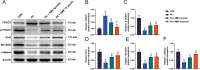
Results: 4MR reduced fasting blood glucose and lipid levels, improved insulin sensitivity, and decreased liver malondialdehyde levels, while increasing catalase and antioxidant enzyme activities. In both mice and HepG2 cells, 4MR increased Sirt1, p-FOXO1, and CAT expression levels and decreased FOXO1 expression levels.
Conclusion: 4MR alleviated hepatic oxidative stress, enhanced insulin sensitivity, and reduced glucose levels.
Keywords: 4′-methoxyresveratrol; anti-oxidative stress; blood glucose; hepatic; insulin resistanc.
© 2025 Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests to declare.
Figures
Similar articles
-
Zinc sulfate improves insulin resistance, oxidative stress and apoptosis in liver tissues of PCOS rats through the NF-κB pathway.Front Endocrinol (Lausanne). 2025 Jun 6;16:1569866. doi: 10.3389/fendo.2025.1569866. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40547527 Free PMC article.
-
Synergies of dibutyl phthalate on high-fat diet can aggravate cardiac fibrosis/dysfunction and the protective effects of vitamin E and salidroside: A molecular toxicological study in Sprague-Dawley rats.Ecotoxicol Environ Saf. 2025 Sep 1;302:118708. doi: 10.1016/j.ecoenv.2025.118708. Epub 2025 Jul 19. Ecotoxicol Environ Saf. 2025. PMID: 40684633
-
Study on the modulation of kidney and liver function of rats with diabetic nephropathy by Huidouba through metabolomics.J Ethnopharmacol. 2025 Jul 24;351:120136. doi: 10.1016/j.jep.2025.120136. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513925
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes.Cochrane Database Syst Rev. 2016 Jun 7;2016(6):CD005542. doi: 10.1002/14651858.CD005542.pub3. Cochrane Database Syst Rev. 2016. PMID: 27272351 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

