Toxicities Associated with Systemic Administration of Interleukin-6 in Wistar Albino Rats
- PMID: 40799355
- PMCID: PMC12341559
- DOI: 10.2147/JEP.S529995
Toxicities Associated with Systemic Administration of Interleukin-6 in Wistar Albino Rats
Abstract
Background: Interleukin-6 is a pleiotropic cytokine being explored in therapy for cancer, trauma, and inflammatory infections, albeit with limited data about its safety. The main aim of this study was to investigate the toxicities associated with systemic administration of interleukin-6 in Wistar albino rats.
Methods: Four groups of rats, each containing six (6) animals received a daily intramuscular dose of 0.3mls of normal saline, 500ng/kg of recombinant interleukin-6, 1000ng/kg of Interleukin-6, and 2000ng/kg of Interleukin-6 for 21 days. On day 22 post-treatment, rats were euthanized, and blood and body organs were collected for analysis. Blood was used to determine liver and renal function, and hematology parameters, while liver and kidney tissue sections were used for histopathological analysis.
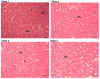
Results: The results revealed that systemic administration of interleukin-6 for 21 days significantly decreased levels of serum creatinine (p<0.00) and serum urea (p<0.01). IL-6 administration had no demonstrable effects on liver function across treatment groups We observed a significant decrease in lymphocytes numbers (p<0.02) across treatment groups when compared to the negative control group. Platelets were significantly elevated in the 100ng/kg treatment groups as compared to the negative control and other treatment groups. Liver and kidney tissue sections for animals that received 500ng/kg of recombinant IL-10 were comparable to those of the negative control and at 1000 and 2000ng/kg, a dose-dependent increase in organ damage was evident.
Conclusion: We demonstrate that systemic administration of recombinant IL-6 at concentrations ranging between 500-1000ng/kg is well tolerated, above this concentration, dose-dependent toxicities and adverse side effects becoming evident. It would be interesting to explore long-term toxicities associated with the systemic administration of IL-6.
Keywords: Wistar albino rats; hepatotoxicity; immunotherapy; interleukin-6; nephrotoxicity.
© 2025 Nabisubi et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures

References
LinkOut - more resources
Full Text Sources

