Distinct microRNA signatures define sporadic PSP-RS and PD in patient-derived midbrain organoids
- PMID: 40799390
- PMCID: PMC12341627
- DOI: 10.1016/j.isci.2025.113162
Distinct microRNA signatures define sporadic PSP-RS and PD in patient-derived midbrain organoids
Abstract
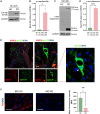
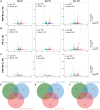
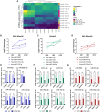
Progressive supranuclear Palsy-Richardson syndrome (PSP-RS) is a rare, rapidly progressive tauopathy often misdiagnosed as Parkinson's disease (PD) due to overlapping clinical features and the lack of reliable molecular biomarkers. To address this need, we generated human midbrain organoids from induced pluripotent stem cells (iPSCs) derived from individuals with sporadic PSP-RS, PD, and healthy controls (HCs), and performed longitudinal small RNA sequencing to profile microRNA (miRNA) signatures. These 3D organoids recapitulated disease-relevant pathologies, including tau hyperphosphorylation in PSP-RS and α-synuclein aggregation in PD. Transcriptomic analysis revealed dynamic, disease-specific miRNA signatures. Notably, miR-5683, miR-873-5p, miR-219b-5p, and miR-219a-2-3p were enriched in PSP-RS, while PD organoids showed increased expression of miR-1-3p and miR-133b. Differential expression analysis identified miR-5683, miR-3085-3p, and miR-124-3p as robust classifiers distinguishing PSP-RS from controls. Our findings support iPSC-derived midbrain organoids as a relevant platform for modeling atypical parkinsonian syndromes and uncovering candidate miRNA biomarkers for early and differential diagnosis.
Keywords: Biological sciences; Cellular neuroscience; Natural sciences; Neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Obeso J.A., Stamelou M., Goetz C.G., Poewe W., Lang A.E., Weintraub D., Burn D., Halliday G.M., Bezard E., Przedborski S., et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017;32:1264–1310. doi: 10.1002/mds.27115. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

