Abnormalities of the endocannabinoid system produce piercing nuclear hernias in migrating cerebral neurons
- PMID: 40799395
- PMCID: PMC12341634
- DOI: 10.1016/j.isci.2025.113188
Abnormalities of the endocannabinoid system produce piercing nuclear hernias in migrating cerebral neurons
Abstract
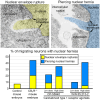
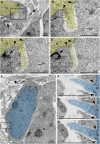
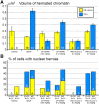
We are reporting powerful streams of chromatin rupturing the nuclear envelope (NE) and the plasma membrane of migrating cerebral neurons in mouse embryos, which we suggest naming "piercing nuclear hernia" (PNH). About 40% of migrating neurons in cannabinoid type 1 receptor knock-out (CB1R-/-) mouse embryos and in wildtype embryos exposed to CB1R agonists show NE rupture and/or PNH. This indicates that deviations from optimal functioning of the endocannabinoid system in under- or over-activity may trigger analogous mechanisms increasing intranuclear pressure and chromatin herniation. The cells from CB1R-/- embryos showed pronounced ultrastructural disorders, such as high volume of herniated chromatin, mitochondrial fission, and negative correlation of the mitochondrial length with the volume of herniated chromatin. Catastrophic rupture of the nuclear and plasma membranes may provoke accidental cell death. At the same time, a fraction of neurons with PNH showed generally normal ultrastructure, which could indicate a mechanism of cell body repair.
Keywords: Biological sciences; Cellular neuroscience; Developmental neuroscience; Natural sciences; Neuroscience.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Mesh versus non-mesh for inguinal and femoral hernia repair.Cochrane Database Syst Rev. 2018 Sep 13;9(9):CD011517. doi: 10.1002/14651858.CD011517.pub2. Cochrane Database Syst Rev. 2018. PMID: 30209805 Free PMC article.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Mahomed A., Girn D., Pattani A., Wells B.K., King C.C., Patel S., Kaur H., Noravian C.M., Sieminski J., Pham C., et al. Cannabinoid receptor type 1 regulates sequential stages of migration and morphogenesis of neural crest cells and derivatives in chicken and frog embryos. J. Morphol. 2023;284 doi: 10.1002/jmor.21606. - DOI - PubMed
LinkOut - more resources
Full Text Sources

