Expert recommendations for biomarker evaluation of advanced non-small cell lung cancer in Thailand
- PMID: 40799428
- PMCID: PMC12337075
- DOI: 10.21037/tlcr-2025-201
Expert recommendations for biomarker evaluation of advanced non-small cell lung cancer in Thailand
Abstract
Background: Growing understanding of the heterogenous molecular profiles of non-small cell lung cancer (NSCLC) has led to changes in the treatment landscape of advanced NSCLC towards precision medicine to target actionable gene alterations. Practical barriers, such as lack of awareness/understanding of biomarkers, suboptimal quality or sample management, inappropriate use of biomarker testing results, limited patient access to biomarker tests and targeted treatments, and reimbursement/payment challenges, hinder the wider adoption of guideline-recommended biomarker testing. Limited reimbursement of targeted therapies is a key consideration for Thai oncologists when making a treatment choice for their patients with advanced NSCLC in Thailand. We aim to assess the current state of biomarker testing and treatment for advanced NSCLC in Thailand and provide recommendations to facilitate timely access to appropriate therapies, enhance patient quality of life, and optimize the use of Thailand's existing healthcare schemes.
Methods: The expert panel comprising one clinical pathologist, three anatomic pathologists, one molecular geneticist, and four medical oncologists convened to review recent literature, discuss current clinical practice, and prioritize essential topics for biomarker assessment and management of advanced NSCLC in Thailand. Following the meeting, further discussions on these prioritized topics were conducted via email, and the recommendations were developed.
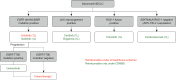
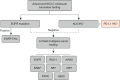
Results: Our recommendations include adopting an exclusionary strategy for biomarker testing, emphasizing the role of a multidisciplinary team (MDT) in managing patients with advanced NSCLC, and underscoring the importance of laboratory accreditation and external quality assurance programs. Additionally, we highlight the need for high-quality data on the local impact of novel treatments to assist policymakers in making these therapies accessible to suitable patients.
Conclusions: By proposing practical strategies tailored to our local healthcare setting, such as exclusionary biomarker testing approach, MDT involvement, and robust quality assurance measures, we provide a roadmap for improving the diagnosis and treatment of advanced NSCLC.
Keywords: Non-small cell lung cancer (NSCLC); biomarker testing; next-generation sequencing (NGS); targeted therapy.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-201/coif). All authors acknowledge In Vivo Communications Asia Pte. Ltd. for editorial assistance which was funded by Amgen Thailand. S.S. reports that he has received honorarium for lectures from Amgen, AstraZeneca, MSD, Novartis, and Takeda; support from the Health Systems Research Institute of Thailand for genetic testing for non-small cell lung cancer in Thai patients; and is the secretary of International Academy of Pathology (IAP)-Thailand Division. Naiyarat Prasongsook reports that he has received speaker honoraria from Amgen, AstraZeneca, BMS, Roche, MSD, Takeda; travel support from AstraZeneca, Roche, MSD. L.T. reports that he has received honoraria from Amgen, Roche, AstraZeneca, MSD, BMS, Johnson & Johnson, Novartis, Eisai, Pfizer, Takeda; travel support from Amgen, MSD, Roche, AstraZeneca. T.R. reports that she has received honoraria from AstraZeneca, Roche, BMS, J&J, Pfizer, Amgen, Takeda, MSD; has participated in data safety monitoring board/advisory board for AstraZeneca, Roche, BMS, J&J, Pfizer, Amgen, Takeda, Yuhan, MSD; and has received grant for Clinical Research from AstraZeneca, Roche, MSD, Yuhan (paid to her institution). Naravat Poungvarin reports that he has received speaker honoraria from Biogenic, AstraZeneca, Pfizer, Amgen, and Roche. V.S. reports that he has received consulting fees from Astellas, AstraZeneca, Merck Sharp & Dohme, Roche, Novartis, Takeda; speaker honoraria from Astellas, AstraZeneca, Merck Sharp & Dohme, Roche, Novartis, Takeda, Pfizer, Eisai; travel support from Eisai, Roche, AstraZeneca, MSD, Celltrion; research support from Astellas, AstraZeneca, Daiichi-Sankyo, Merck Sharp & Dohme, Novartis, Roche, Takeda. The authors have no other conflicts of interest to declare.
Figures
References
-
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. 2024. Lyon: International Agency for Research on Cancer. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/764-thail...
-
- Srisam-Ang K, Podhipak A, Narksawat K, et al. Survival of patients with advanced non-small-cell lung cancer at Ubon Ratchathani Cancer Center, Thailand. Southeast Asian J Trop Med Public Health 2005;36:994-1006. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
