A preliminary analysis of integrating thymosin α1 into concurrent chemoradiotherapy and consolidative immunotherapy in unresectable locally advanced non-small cell lung cancer
- PMID: 40799433
- PMCID: PMC12337057
- DOI: 10.21037/tlcr-2025-190
A preliminary analysis of integrating thymosin α1 into concurrent chemoradiotherapy and consolidative immunotherapy in unresectable locally advanced non-small cell lung cancer
Abstract
Background: Concurrent chemoradiotherapy (CCRT) followed by consolidative immunotherapy represents the standard of care for unresectable locally advanced non-small cell lung cancer (LA-NSCLC), but critical challenges persist: a considerable number of patients discontinue consolidative immune checkpoint inhibitors (ICIs) due to treatment-related pneumonitis and lymphopenia, while "cold" tumor microenvironments further limit immunotherapy efficacy. Thymosin α1 (Tα1) is a pleiotropic immunomodulator that has been associated with infection prevention and the regulation of immune cells. Thus, we designed this retrospective study to investigate the therapeutic effect of integrating Tα1 into CCRT followed by consolidative immunotherapy in patients diagnosed with unresectable LA-NSCLC.
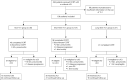
Methods: A retrospective analysis was conducted on a total of 196 patients with unresectable stage IIIA-IIIC LA-NSCLC treated from January 1, 2020, to May 31, 2023. All patients received CCRT (60-64 Gy total irradiation dose, weekly concurrent docetaxel and cisplatin) with or without consolidative nivolumab. According to the use of Tα1, patients were classified into 3 groups: non-Tα1 group, patients who did not receive Tα1; short-term Tα1 group, receipt of Tα1 (1.6 mg) once a week from the beginning of treatment until the end of CCRT; long-term Tα1 group, receipt of Tα1 (1.6 mg) once a week from the beginning of treatment until 12 months post-CCRT. The primary objective was progression-free survival (PFS). The secondary objectives included overall survival (OS), pneumonitis, circulating lymphocyte count and interleukin-6 (IL-6) levels. Pretreatment biopsy samples were collected to evaluate the potential influence of somatic mutations on treatment outcomes.
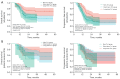
Results: The non-Tα1, short-term Tα1, and long-term Tα1 groups included 48, 101, and 47 patients, respectively. Following CCRT, 77.1%, 75.2%, and 93.6% of patients in the respective groups were eligible for consolidative nivolumab (P=0.03). Median PFS was 14.6 months [95% confidence interval (CI): 11.9-17.3] for the non-Tα1 group, 16.0 months (95% CI: 13.2-18.8) for the short-term Tα1 group, and not reached for the long-term Tα1 group (P=0.03). Median OS was 20.0 months (95% CI: 16.1-23.9) for the non-Tα1 group, 27.6 months (95% CI: 13.8-41.3) for the short-term Tα1 group, and not reached in the long-term Tα1 group (P=0.01). The long-term Tα1 group experienced significantly lower rates of grade ≥2 pneumonitis (35.4% in non-Tα1, 14.5% in long-term Tα1 groups, P=0.02), and lower rates of lymphopenia at 6 months post-CCRT (55.8% in non-Tα1, 30.9% in short-term Tα1, and 22.5% in long-term Tα1 groups, P=0.01). At 2 months post-CCRT, the median IL-6 level in the non-Tα1 group (8.14 pg/mL) was significantly higher than that in the long-term Tα1 group (4.92 pg/mL, P=0.03).
Conclusions: Integrating Tα1 into CCRT and consolidative immunotherapy could have a synergistic effect in patients with LA-NSCLC. This combination may enhance survival outcomes and reduce treatment-related toxicity. Further randomized trial is warranted for validation.
Keywords: Locally advanced non-small cell lung cancer (LA-NSCLC); concurrent chemoradiotherapy (CCRT); immunotherapy; thymosin α1 (Tα1).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-190/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Evaluation of Radiation Pneumonitis in a Phase 2 Study of Consolidation Immunotherapy With Nivolumab and Ipilimumab or Nivolumab Alone Following Concurrent Chemoradiation Therapy for Unresectable Stage IIIA/IIIB Non-Small Cell Lung Cancer.Int J Radiat Oncol Biol Phys. 2025 Mar 1;121(3):720-727. doi: 10.1016/j.ijrobp.2024.09.050. Epub 2024 Oct 26. Int J Radiat Oncol Biol Phys. 2025. PMID: 39490906 Clinical Trial.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2022 Aug 22;8(8):CD010260. doi: 10.1002/14651858.CD010260.pub3. Cochrane Database Syst Rev. 2022. PMID: 35994243 Free PMC article.
References
LinkOut - more resources
Full Text Sources
