Electrochemical Ammoxidation of Unprotected Glycosides
- PMID: 40799488
- PMCID: PMC12337100
- DOI: 10.1021/acselectrochem.5c00111
Electrochemical Ammoxidation of Unprotected Glycosides
Abstract
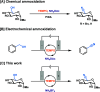
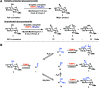
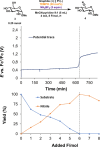
Cyano-sugars are useful carbohydrate building blocks. Here, we report an electrocatalytic method to oxidize the primary alcohols of unprotected glycosides to the corresponding nitrile using 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) as a mediator and ammonium tetrafluoroborate as the ammonia source. Monosaccharides and disaccharides undergo successful reactions in an acetonitrile/pyridine solvent mixture at room temperature under constant current electrolysis.
Keywords: Electrocatalytic oxidation; TEMPO; ammoxidation; carbohydrates; nitriles.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Maßmann S. C., Metselaar G. A., van Dijken D. J., van den Berg K. J., Witte M. D., Minnaard A. J.. Regioselective Palladium-Catalysed Aerobic Oxidation of Dextran and Its Use as a Bio-Based Binder in Paperboard Coatings. Green Chem. 2024;26(7):4005–4012. doi: 10.1039/D3GC04204A. - DOI - PMC - PubMed
-
- Horton, D. The Development of Carbohydrate Chemistry and Biology. Carbohydrate Chemistry, Biology and Medical Applications; Elsevier, 2008; pp 1–28. 10.1016/B978-0-08-054816-6.00001-X. - DOI
LinkOut - more resources
Full Text Sources
