This is a preprint.
Mechanistic Insights into Dual-Active Liver and Blood-Stage Antiplasmodials
- PMID: 40799604
- PMCID: PMC12340835
- DOI: 10.1101/2025.08.06.666330
Mechanistic Insights into Dual-Active Liver and Blood-Stage Antiplasmodials
Update in
-
Mechanistic insights into dual-active liver and blood-stage antiplasmodials.mBio. 2026 Jan 14;17(1):e0242325. doi: 10.1128/mbio.02423-25. Epub 2025 Nov 24. mBio. 2026. PMID: 41277867 Free PMC article.
Abstract
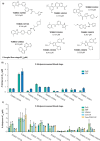
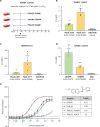
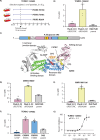
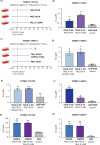
The identification of novel antimalarials with activity against both the liver and blood stages of the parasite lifecycle would have the dual benefit of prophylactic and curative potential. However, one challenge of leveraging chemical hits from phenotypic screens is subsequent target identification. Here, we use in vitro evolution of resistance to investigate nine compounds from the Tres Cantos Antimalarial Set (TCAMS) with dual liver and asexual blood stage activity. We succeeded in eliciting resistance to four compounds, yielding mutations in acetyl CoA synthetase (AcAS), cytoplasmic isoleucine tRNA synthetase (cIRS), and protein kinase G (PKG) respectively. Using a combination of CRISPR editing and in vitro activity assays with recombinant proteins, we validate these as targets for TCMDC-125075 (AcAS), TCMDC-124602 (cIRS), and TCMDC-141334 and TCDMC-140674 (PKG). Notably, for the latter two compounds, we obtained a T618I mutation in the gatekeeper residue of PKG, consistent with direct interaction with the active site, which we modelled with molecular docking. Finally, we performed cross-resistance evaluation of the remaining five resistance-refractory compounds using the Antimalarial Resistome Barcode sequencing assay (AReBar), which examined a pool of 52 barcoded lines with mutations covering >30 common modes of action. None of the five compounds where in vitro evolution of resistance was not successful yielded validated hits using AReBar, indicating they likely act via novel mechanisms and may be candidates for further exploration.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures

References
-
- WHO, World Malaria Report., in https://www.who.int/publications/i/item/9789240104440:. 2024.
-
- Sambo L.G., Defining and defeating the intolerable burden of malaria III. Progress and perspectives. The American Journal of Tropical Medicine and Hygiene, 2007. 77(6_Suppl): p. iii–iii.
-
- Noedl H., et al. , Evidence of artemisinin-resistant malaria in western Cambodia. New England Journal of Medicine, 2008. 359(24): p. 2619–2620. - PubMed
-
- Rosenthal P.J., et al. , The emergence of artemisinin partial resistance in Africa: how do we respond? The Lancet Infectious Diseases, 2024. 24(9): p. e591–e600. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
