A 7T interleaved fMRS and fMRI study on visual contrast dependency in the human brain
- PMID: 40799712
- PMCID: PMC12007529
- DOI: 10.1162/imag_a_00031
A 7T interleaved fMRS and fMRI study on visual contrast dependency in the human brain
Abstract
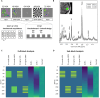
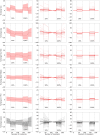
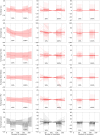
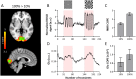
Introduction: Functional magnetic resonance spectroscopy (fMRS) is a non-invasive technique for measuring dynamic changes in neurometabolites. While previous studies have observed concentration changes in metabolites during neural activation, the relationship between neurometabolite response and stimulus intensity and timing requires further investigation. To address this, we conducted an interleaved fMRS and functional magnetic resonance imaging (fMRI) experiment using a visual stimulus with varying contrast levels. Methods: A total of 20 datasets were acquired on a 7T MRI scanner. The visual task consisted of two STIM blocks (30 s/20 s ON/OFF, 4 min), with 10% or 100% contrast, interleaved with a 5 min REST block. A dynamic fitting approach was used for fMRS data analysis. For metabolite level changes, the STIM conditions were modeled in two different ways: either considering the full STIM block as active condition (full-block model) or only modeling the ON blocks as active condition (sub-block model). For linewidth changes due to the BOLD effect, STIM conditions were modeled using the sub-block model. Results: For both models, we observed significant increases in glutamate levels for both the 10% and 100% visual contrasts, but no significant difference between the contrasts. Decreases in aspartate, and glucose, and increases in total N-acetylaspartate and total creatine were also detected, although less consistently across both 10% and 100% visual contrasts. BOLD-driven linewidth decreases and fMRI-derived BOLD increases within the MRS voxel were observed at both 10% and 100% contrasts, with larger changes at 100% compared to 10% in the fMRI-derived BOLD only. We observed a non-linear relation between visual contrast, the BOLD response, and the glutamate response. Conclusion: Our study highlights the potential of fMRS as a complementary technique to BOLD fMRI for investigating the complex interplay between visual contrast, neural activity, and neurometabolism. Future studies should further explore the temporal response profiles of different neurometabolites and refine the statistical models used for fMRS analysis.
Keywords: 7T; functional magnetic resonance imaging; functional magnetic resonance spectroscopy; visual contrast.
© 2023 Massachusetts Institute of Technology.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Arteaga de Castro, C. S., Boer, V. O., Andreychenko, A., Wijnen, J. P., van der Heide, U. A., Luijten, P. R., & Klomp, D. W. (2013). Improved efficiency on editing MRS of lactate and γ-aminobutyric acid by inclusion of frequency offset corrected inversion pulses at high fields. NMR in Biomedicine, 26(10), 1213–1219. 10.1002/nbm.2937 - DOI - PubMed
-
- Bednařík, P., Tkáč, I., Giove, F., DiNuzzo, M., Deelchand, D. K., Emir, U. E., Eberly, L. E., & Mangia, S. (2015). Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 35(4), 601–610. 10.1038/jcbfm.2014.233 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
